Transmissible News
Category Archives:Transmissible News
A day to remember – Andrija Štampar’s birthday
 It was 129 years ago today……
It was 129 years ago today……
Andrija was born 1 September 1888 in Brodski Drenovac, in modern Požega-Slavonia County. He enrolled at the Medical School in Vienna in 1906, which was at the time the most important medical center in the world. On 23 December 1911, he was awarded the title of Doctor of Universal Medicine.
In 1919, he attended the Congress of Inter-Allied Countries for Social Hygiene in Paris giving a lecture on children’s health. It showed at that time that he had a clear concept of organizing the public health service. Andrija Štampar is universally known as “the man of action”.
At the young age of 31 he became principal of the former Yugoslav Health Service in Belgrade (Beograd). Thanks to Štampar’s endeavours, a special Institute of Social Medicine was founded affiliated with the University of Zagreb School of Medicine.
From 1931 to 1933, Štampar was permanently employed as the expert of the League of Nation’s Health Organization. The Health Organization sent him as an advisor to help the Chinese health administration in the control of the mass infectious diseases that cropped up after devastating floods in 1931.
Dr. Štampar has come to China to help our Government in its work on reconstruction based on the plan of technical cooperation with the League of Nations. He went round several provinces, from Kansu and Shanghai in the West to Kwangtung and Kwangsi in the South, and made a valuable contribution to the reconstruction of our villages, especially in the field of rural health protection services. On his departure we wish to give this to him as a remembrance of his work in China, hoping he will come to visit us again.
— Ching Feng
During the International Health Conference in New York in the summer of 1946 the draft of the World Health Organization (WHO) was accepted. The First World Health Assembly was called with the ratification of the WHO Constitution. It was in session from 24 June to 24 July 1948. in Geneva, Štampar was elected as the first President of the Assembly unanimously. At the 8th regular session of WHO in Mexico City, in 1955, Štampar was awarded the Leon Bernard Foundation Prize and Medal, the greatest international recognition of merit in the field of social medicine.
Andrija Štampar founded School of Public Health in Zagreb in 1927. He became the Dean of the Medical School of Zagred University for the academic year 1940/41. With the energy so characteristic of him, he set to work on the reform of medical training. During the German occupation of WWII, Stampar was arrested and interned. On his return in May 1945, he resumed his duty as Professor of Hygiene and Social Medicine at the Medical School and became head of the School of Public Health in Zagreb.
Štampar was the Rector of Zagreb University for the academic year 1945/46. In 1952, he was again elected the Dean of the Medical School, for 5 years consecutively. He also had an important role in founding of the Medical School at Rijeka in 1955.
References:
European Antibiotic Awareness Day 2017
EAD
[aesop_video src=”self” hosted=”https://www.transmissible.eu/wp-content/uploads/2019/04/A-bottle-of-white-pills-falling-and-spilling-in-slow-motion-studio-shot.mp4″ align=”center” disable_for_mobile=”on” poster_frame=”bosmana” loop=”on” controls=”off” autoplay=”on” mute=”off” viewstart=”on” viewend=”on” overlay_content=”
The EAAD is organized each year on 18 November to raise awareness about the threat to public health of antibiotic resistance and the importance of prudent antibiotic use.
The latest data confirm that across the European Union the number of patients infected by resistant bacteria is increasing and that antibiotic resistance is a major threat to public health.
Prudent use of antibiotics can help stop resistant bacteria from developing and help keep antibiotics effective for the use of future generations.” revealfx=”off” overlay_revealfx=”off”]
Joint Transmissible & Trimension workshop for Communicable Disease Control Training
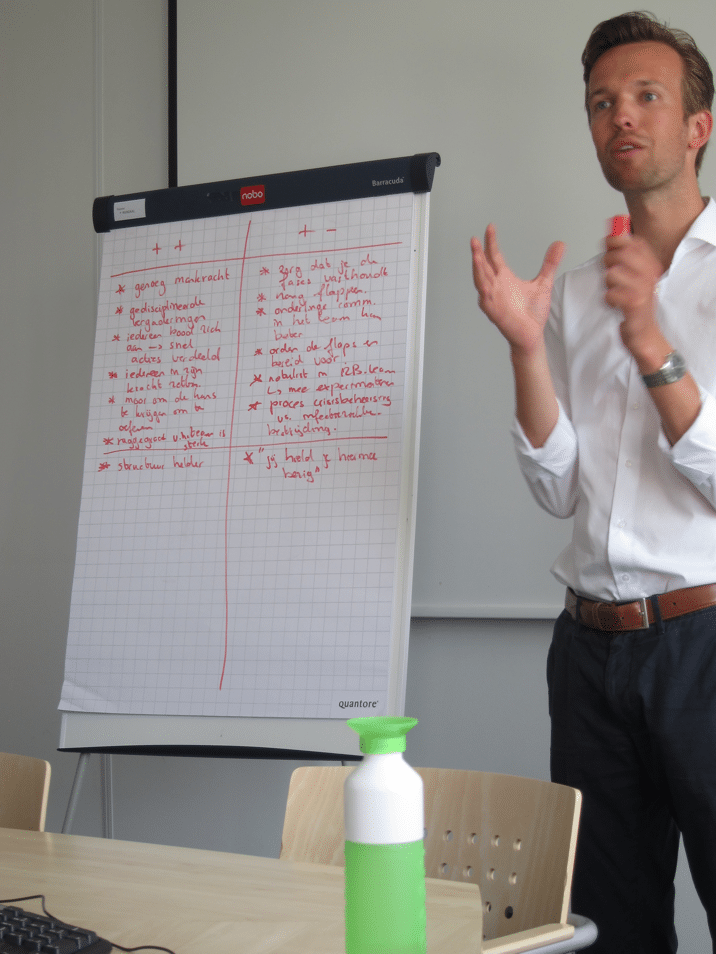 A large group of people gets contaminated with a virulent pathogen, and regular treatment is ineffective. To make matters worse, the control measures are interrupted by increasing civil unrest. The Communicable Disease Control team of the Public Health Service ‘Hollands-Midden’, dared to engage in this scenario during a training workshop.
A large group of people gets contaminated with a virulent pathogen, and regular treatment is ineffective. To make matters worse, the control measures are interrupted by increasing civil unrest. The Communicable Disease Control team of the Public Health Service ‘Hollands-Midden’, dared to engage in this scenario during a training workshop.
As per request of the Public Health Service, communicable disease Control consultant Arnold Bosman (Transmissible) and Trimension trainer Arthur van Lohuijzen got together to organise and animate this workshop. Arnold was responsible for detailing the case finding and epidemiological investigation. Arthur put this in the context of crisis management. The participants showed great engagement to identify the origin of the disease, in order to block further spread. In addition, the participant could use several interim evaluations to review their approach. It turned out a challenge to combine the structured approach according to the principles of crisis management with the required outbreak response.
Both trainers consider this collaboration between Transmissible and Trimension as a clear success and aim for continuing this training offer. Are you interested in to receive more details on this workshop, feel free to contact:
Arthur van Lohuijzen – arthur@trimension.nl
Arnold Bosman – Arnold.Bosman@Transmissible.EU
For more Transmissible training, read this page.
A day to remember – Alexander Fleming

Sir Alexander Fleming, (6 August 1881 – 11 March 1955) was a Scottish biologist, pharmacologist and botanist who discovered Penicillin. (Photo by Universal History Archive/UIG via Getty Images)
It was 136 years ago today…
that Alexander Fleming was born, on August 6, 1881, in Ayrshire, Scotland. He was the third of the four children of farmer Hugh Fleming (1816–1888) and his wife Grace Stirling Morton.
After working in a shipping office for four years, the twenty-year-old Fleming inherited some money from an uncle, John Fleming. His elder brother, Tom, was already a physician and suggested to him that he should follow the same career, and so in 1903, the younger Alexander enrolled at St Mary’s Hospital Medical School in Paddington; he qualified with an MBBS degree from the school with distinction in 1906. Het joined the research department at St Mary’s, where he became assistant bacteriologist to Sir Almroth Wright, a pioneer in vaccine therapy and immunology.
In 1908, he gained a BSc degree with Gold Medal in Bacteriology, and became a lecturer at St Mary’s until 1914. Fleming served throughout World War I as a captain in the Royal Army Medical Corps, and was Mentioned in Dispatches. He and many of his colleagues worked in battlefield hospitals at the Western Front in France. In 1918 he returned to St Mary’s Hospital, where he was elected Professor of Bacteriology of the University of London in 1928.
Work before penicillin
During World War I, Fleming witnessed the death of many soldiers from sepsis resulting from infected wounds. Antiseptics, which were used at the time to treat infected wounds, often worsened the injuries. In an article he submitted for the medical journal The Lancet during World War I, Fleming described an ingenious experiment, which he was able to conduct as a result of his own glass blowing skills, in which he explained why antiseptics were killing more soldiers than infection itself during World War I. Antiseptics worked well on the surface, but deep wounds tended to shelter anaerobic bacteria from the antiseptic agent, and antiseptics seemed to remove beneficial agents produced that protected the patients in these cases at least as well as they removed bacteria, and did nothing to remove the bacteria that were out of reach. Sir Almroth Wright strongly supported Fleming’s findings, but despite this, most army physicians over the course of the war continued to use antiseptics even in cases where this worsened the condition of the patients.
At St Mary’s Hospital Fleming continued his investigations into antibacterial substances. Testing the nasal secretions from a patient with a heavy cold, he found that nasal mucus had an inhibitory effect on bacterial growth. This was the first recorded discovery of lysozyme, an enzyme present in many secretions including tears, saliva, skin, hair and nails as well as mucus. Although he was able to obtain larger amounts of lysozyme from egg whites, the enzyme was only effective against small counts of harmless bacteria, and therefore had little therapeutic potential.
Accidental discovery
When I woke up just after dawn on September 28, 1928, I certainly didn’t plan to revolutionise all medicine by discovering the world’s first antibiotic, or bacteria killer. But I suppose that was exactly what I did.
— Alexander Fleming
By 1927, Fleming had been investigating the properties of staphylococci. He was already well-known from his earlier work, and had developed a reputation as a brilliant researcher, but his laboratory was often untidy. On 3 September 1928, Fleming returned to his laboratory having spent August on holiday with his family. Before leaving, he had stacked all his cultures of staphylococci on a bench in a corner of his laboratory. On returning, Fleming noticed that one culture was contaminated with a fungus, and that the colonies of staphylococci immediately surrounding the fungus had been destroyed, whereas other staphylococci colonies farther away were normal, famously remarking “That’s funny”.
Fleming grew the mould in a pure culture and found that it produced a substance that killed a number of disease-causing bacteria. He identified the mould as being from the Penicillium genus, and, after some months of calling it “mould juice”, named the substance it released penicillin on 7 March 1929.
He investigated its positive anti-bacterial effect on many organisms, and noticed that it affected bacteria such as staphylococci and many other Gram-positive pathogens that cause scarlet fever, pneumonia, meningitis and diphtheria, but not typhoid fever or paratyphoid fever, which are caused by Gram-negative bacteria, for which he was seeking a cure at the time. It also affected Neisseria gonorrhoeae, which causes gonorrhoea although this bacterium is Gram-negative.
Not much attention…
Fleming published his discovery in 1929, in the British Journal of Experimental Pathology, but little attention was paid to his article. Fleming continued his investigations, and found that cultivating penicillium was quite difficult, and that after having grown the mould, it was even more difficult to isolate the antibiotic agent. Fleming’s impression was that because of the problem of producing it in quantity, and because its action appeared to be rather slow, penicillin would not be important in treating infection. Fleming also became convinced that penicillin would not last long enough in the human body (in vivo) to kill bacteria effectively. Many clinical tests were inconclusive, probably because it had been used as a surface antiseptic.
 In the 1930s, Fleming’s trials occasionally showed more promise, and he continued, until 1940, to try to interest a chemist skilled enough to further refine usable penicillin. Fleming finally abandoned penicillin, and not long after he did, Howard Florey and Ernst Boris Chain at the Radcliffe Infirmary in Oxford took up researching and mass-producing it, with funds from the U.S. and British governments. They started mass production after the bombing of Pearl Harbor. By D-Day in 1944, enough penicillin had been produced to treat all the wounded in the Allied forces.
In the 1930s, Fleming’s trials occasionally showed more promise, and he continued, until 1940, to try to interest a chemist skilled enough to further refine usable penicillin. Fleming finally abandoned penicillin, and not long after he did, Howard Florey and Ernst Boris Chain at the Radcliffe Infirmary in Oxford took up researching and mass-producing it, with funds from the U.S. and British governments. They started mass production after the bombing of Pearl Harbor. By D-Day in 1944, enough penicillin had been produced to treat all the wounded in the Allied forces.
Fleming was said to be “a reticent and rather taciturn man, with great independence of mind and strength of character”. He was a keen observant of nature and everything around him, always ready to devise and test new methods for studying germs in the lab. He accepted the many honors that were bestowed upon him with modesty. It seemed that simple tributes touched him most, such as the letter of some poor person who had benefitted from penicillin.
Fleming died on 11 March 1955, at his home in London of a heart attack. He was buried in St Paul’s Cathedral
References:
- Alexander Fleming, in: Wikipedia, accessed 6 August 2017
- Robert Cruickshank, Obituary of Alexander Fleming. In: Nature, nr 4459, April 16,1955, P663
A day to remember: Daniel Elmer Salmon
 It was 167 years ago today….
It was 167 years ago today….
when Daniel Elmer Salmon (July 23, 1850 – August 30, 1914) was a veterinary surgeon. He earned the first D.V.M. degree awarded in the United States, and spent his career studying animal diseases for the U.S. Department of Agriculture. He gave his name to the Salmonella genus of bacteria, which was discovered by an assistant, and named in his honor.
Salmon was born in Mount Olive Township, New Jersey.[1] Dr. Salmon’s father, Daniel L. Salmon, died in 1851 and his mother, Eleanor Flock Salmon, died in 1859, leaving him an orphan at the age of 8. He was then raised by his second cousin, Aaron Howell Salmon and spent time working both on Aaron’s farm and as a clerk in a country store. His early education was at the Mount Olive District School, Chester Institute, and Eastman Business College. He then attended Cornell University and graduated with the degree of Bachelor of Veterinary Medicine in 1872. After an additional four years of study, in veterinary health and science, he was awarded the professional degree of Doctor of Veterinary Medicine from Cornell in 1876, the first D.V.M. degree granted in the United States. Toward the end of his career at Cornell, he studied at the Alfort Veterinary School in Paris, France.
Dr. Salmon opened a veterinary practice in Newark, New Jersey in 1872 and subsequently moved to Asheville, North Carolina in 1875 due to his health. In 1877 he gave a series of lectures at the University of Georgia on the topic of veterinary science. He worked for the State of New York, studying diseases in swine and for the United States Department of Agriculture studying animal diseases in the southern states. In 1883 he was asked to establish a veterinary division within the Department of Agriculture. It became the Bureau of Animal Industry and he served as its chief from 1884 to December 1, 1905. Under his leadership, the Bureau eradicated contagious pleural-pneumonia of cattle in the United States, studied and controlled Texas fever (Babesia), put in place the federal meat inspection program, began inspecting exported livestock and the ships carrying them, began inspecting and quarantining imported livestock, and studied the effect of animal diseases on public health. In 1906 he established the veterinary department at the University of Montevideo, Uruguay and was its head for five years. He returned to the United States in 1911 and concentrated on veterinary work in the western region of the country.
Salmonella is a genus of microorganisms named after him in Modern Latin in 1900 by J. Lignières, although the man who actually discovered and named the first strain, Salmonella cholerae suis, was Theobald Smith, Dr. Salmon’s research assistant, who isolated the bacterium in 1885.
Since that time, more than 2,000 subtypes have been identified.
Daniel Salmon died of pneumonia August 30, 1914, in Butte, Montana and is buried in Washington, D.C.
References:
A day to remember: Austin Bradford Hill – father of causation viewpoints
 It was 120 years ago, today
It was 120 years ago, today
When Austin Bradford Hill (8 July 1897 – 18 April 1991) was born in London. He was an English epidemiologist and statistician, pioneered the randomized clinical trial and, together with Richard Doll, demonstrated the connection between cigarette smoking and lung cancer. Hill is widely known for pioneering the “Bradford Hill criteria” for determining a causal association; however, that seems to be a falsification from his personal views. He never seems to have seen these as ‘criteria’, yet merely ‘viewpoints’.
As a child, he lived at the family home, Osborne House, Loughton, Essex; he was educated at Chigwell School, Essex, and later served as a pilot in the First World War but was invalided out when he contracted tuberculosis. Two years in hospital and two years of convalescence put a medical qualification out of the question and he took a degree in economics by correspondence at London University.
In 1922 Hill went to work for the Industry Fatigue Research Board. He was associated with the medical statistician Major Greenwood and, to improve his statistical knowledge, Hill attended lectures by Karl Pearson. When Greenwood accepted a chair at the newly formed London School of Hygiene and Tropical Medicine, Hill moved with him, becoming Reader in Epidemiology and Vital Statistics in 1933 and Professor of Medical Statistics in 1947.
Hill had a distinguished career in research and teaching and as author of a very successful textbook, Principles of Medical Statistics, but he is famous for two landmark studies. He was the statistician on the Medical Research Council Streptomycin in Tuberculosis Trials Committee and their study evaluating the use of streptomycin in treating tuberculosis, is generally accepted as the first randomised clinical trial. The use of randomisation in agricultural experiments had been pioneered by Ronald Aylmer Fisher. The second study was rather a series of studies with Richard Doll on smoking and lung cancer. The first paper, published in 1950, was a case-control study comparing lung cancer patients with matched controls. Doll and Hill also started a long-term prospective study of smoking and health. This was an investigation of the smoking habits and health of 40,701 British doctors for several years (British doctors study). Fisher was in profound disagreement with the conclusions and procedures of the smoking/cancer work and from 1957 he criticised the work in the press and in academic publications.
Hill was made a fellow of the Royal Society in 1954. Fisher was actually one of the proposers. The certificate of election read:
Has, by the application of statistical methods, made valuable contributions to our knowledge of the incidence and aetiology of industrial diseases, of the effects of internal migration upon mortality rates, and of the natural and experimental epidemiology of various infections, for example of the risks of an attack of poliomyelitis following inoculation procedures and of the risk of congenital abnormalities being precipitated by maternal rubella in the pregnant woman. Since the war he has demonstrated in an exact and controlled field survey the association between cigarette smoking and the incidence of cancer of the lung, and has been the leader in the development in medicine of the precise experimental methods now used nationally and internationally in the evaluation of new therapeutic and prophylactic agents.
In 1950–52 Hill was president of the Royal Statistical Society and was awarded its Guy Medal in Gold in 1953. He was knighted in 1961. On Hill’s death Peter Armitage wrote,
“to anyone involved in medical statistics, epidemiology or public health, Bradford Hill was quite simply the world’s leading medical statistician.”
Work on Causation
Bradford Hill set out nine viewpoint on causality: strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, experimental evidence, and analogy. While these viewpoints are helpful when considering cause and effect, he insisted that
“none of [his] nine viewpoints can bring indisputable evidence for or against the cause-and effect hypothesis”.
What they can do, with greater or lesser strength, is to help epidemiologists make up their minds on the fundamental question – Is there any other way of explaining the set of facts before them? Is there any other answer equally, or more, likely than cause and effect?
It is important to keep in mind that most judgments of cause in epidemiology are tentative and should remain open to change with new evidence. It is important to be remain critical, to aim always for stronger evidence, and to keep an open mind. Checklists of causal criteria should not replace critical thinking.
“The world is richer in associations than meanings, and it is the part of wisdom to differentiate the two.”
John Barth, novelist.
References:
Day 2 – Digital Health Conference notes
Digital support to humanitarian aid
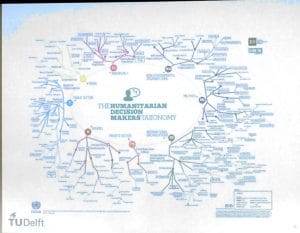
Day 2 started with a keynote presentation by professor Tina Gomes of Delft Technical University. Information systems need to address decision makers needs, which is easier said than done. Because: who are they? The taxonomy map of decision makers in humanitarian aid is extremely complex, and covers levels from grass roots (field) level, up to international organisations. Understanding this mapping is a key starting point in system design.
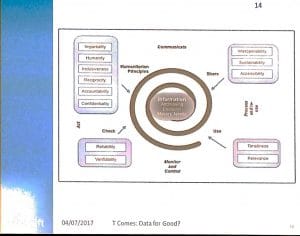
Dr. Gomes made the point that the cycle starts with preparedness, where issues of interoperability, sustainability and accessibility are key to emergency aid system design. At the development stage as well as in the stage of monitor and control, the issues of timeliness and relevance need to guide the functionality. Reliability and verifiability come into view at this stage.
When actions are taken, these need to follow the humanitarian principles of impartiality, humanity, inclusiveness, reciprocity, accountability and confidentiality. System designers will have to take these principles into the system design as well.
When you provide information to decision makers, then YOU become part of the decision making process.
This is a point that Dr. Gomes communicated clearly to the audience, with the view that technology does have an impact.
 Developing new systems for humanitarian aid is a complex matter, and the worst thing one could do, is rolling out a new system during a crisis. Gomes illustrated this with an example from the 2014-2015 Ebola response.
Developing new systems for humanitarian aid is a complex matter, and the worst thing one could do, is rolling out a new system during a crisis. Gomes illustrated this with an example from the 2014-2015 Ebola response.
Implementing new information technology in low and middle income countries for response to humanitarian crisis is complicated by many factors, not in the least the inverse relationship of ID-address density and vulnerability of populations.
Innovation awards
The second part of the morning included a series of elevator pitches of candidates for the innovation awards. More about that in an update of this post
Digital Health Conference 2017 – Day 1
From 3-5 July 2017, the 11th International Digital Health Conference is held in London, hosted by UCL. This year is the first ‘independent’ year, and therefore exciting to see how this event performs without being embedded in a larger IT conference.
The conference covers a wide spectrum of subjects, including communities of practice and social networks, analytics and engagement with tracking and monitoring wearable devices, big data, public health surveillance, persuasive technologies, epidemic intelligence, participatory surveillance, disaster and emergency medicine, serious games for public health interventions, and automated early identification of health threats and response.
The aim of the conference is to bring together public health agencies (WHO, ECDC, CDC, PHE) and computer science and IT and MedTech industry to cross-fertilize ideas and drive this growing interdisciplinary discipline.
The theme of this year is ’emergency and humanitarian medicine’.
The first day was opened by Oliver Morgan (WHO) with a presentation on the new Health Emergencies structure, and how this links to digital health. In less than one hour, Dr. Morgan gave an impressive overview of the global activities of the WHO in this area, and how IT tools and infrastructure play a vital role. The role of WHO in developing new global digital tools for core functions such as surveillance, early warning, epidemic intelligence and field investigations was very well illustrated.
Other parts of the programme on day 1 included a session on Digital Tools in Practice (which I had the privilege to moderate), poster presentations, a debate session on funding and impact on digital health, parallel sessions on online communities and modeling.
Looking back on Day 1, we see a rich diversity of digital health topics that illustrates how much this field is alive, even though the theme of this year may not have been that obvious in all sessions.
Follow tweets on this conference:
and
Fanny Hesse – the woman who made microbiology possible

Fanny Hesse
Fanny Hesse (Born Angelina Fanny Elishemius, June 22, 1850 – December 1, 1934) is best known for her work in microbiology alongside her husband, Walther Hesse. Together they were instrumental in developing Agar as a medium for culturing microorganisms. She was born in 1850 in New York City to Gottfried Elishemius, a wealthy import merchant, and his wife, Cecile Elise. Fanny met her husband and research partner Walther Hesse in 1872 while in Germany. They were engaged in 1873 and married in 1874 in Geneva.[1]
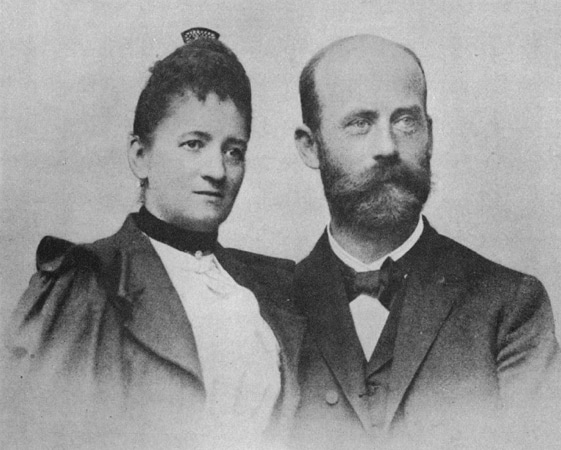
Fanny and Walter Hesse
In 1881, she worked for her husband as a technician in the laboratory of German physician and microbiologist Robert Koch. Hesse, working unpaid, would make drawings for her husband’s publications.[1] At the time, Koch was desperately searching for a suitable medium to grow bacterial cultures. He originally used potato slices, yet not all bacteria would grow on that surface. Then he used gelatin broths, yet during warm weather, these would liquefy and become all gooey. Besides, several bacteria used enzymes to break down the gelatin.[2]
One day in 1881, while eating lunch, Walter asked Fanny about the jellies and puddings that she made and how they managed to stay gelled even in warm weather. Fannie told him about how she learned about the seaweed product, agar-agar, from a Dutch neighbor of hers while she was growing up in New York City. Her neighbor had emigrated from Indonesia, where it was the local custom to use agar in their cooking. Fannie suggested that they try this out in their laboratory. The rest is history. Agar turned out to be an ideal gelling agent that stayed firm even in the incubator and could not be digested by any bacterial enzymes. Walter Hesse notified Koch of this new technique, who immediately added agar to his nutrient broths. [2]
Lab work can be a lot like cooking. You have to follow directions to measure, mix, and heat different chemicals to the right temperature to get the desired result.[3]
This led to Koch using agar to cultivate the bacteria that cause tuberculosis. While Koch, in an 1882 paper on tuberculosis bacilli, mentioned he used agar instead of gelatin, he did not credit Fanny or Walther Hesse or mention why he made the switch. Fanny Hesse’s suggestion never resulted in financial benefit for the Hesse family.[1]
References:
A day to remember: William M. Haenszel
 It was 106 years ago today when William Haenszel was born in Rochester, New York.
It was 106 years ago today when William Haenszel was born in Rochester, New York.
William Manning Haenszel (June 19, 1910 – March 13, 1998) was an American epidemiologist who developed the first national system to track cancer cases and their possible causes (Surveillance, Epidemiology, and End Results or SEER). He was an elected fellow of the American Statistical Association, the American Public Health Association, and the American Association For the Advancement of Science. He worked at the National Cancer Institute from 1952 to 1976, when he became a Professor of Epidemiology at the University of Illinois. With Nathan Mantel, he co-authored the Mantel-Haenszel statistical test for omitted variables.[1]
In the 1970’s, when the Nixon Administration declared a ”war on cancer,” William Haenszel, as chief of biometry — the statistical analysis of biological data — set out to record individual cases, to track them from diagnosis to death, and to synthesize the data at the N.C.I., in Bethesda, Md., to learn about potential causes. SEER — which he started in 1973 — is probably the largest registry for any one disease in the world, said Dr. Earl S. Pollack, who was chief of biometry at the institute after Haenszel. [2]
“Mr. Haenszel provided the intellectual foundation for what epidemiologists do on a day-to-day basis in the study of causes of disease,”
”We can make the right inferences today because of his insights.”
Dr. Jack H. Goldberg
Haenszel was widely known for migrant studies in the 1950’s and 1960’s. In one project, he showed that the high stomach cancer rates in Japan were no longer found in the Japanese who migrated to Hawaii. He identified the role of diet as a possible cause of stomach cancer. In a 1959 paper, written with Nathan Mantel, also of the cancer institute, Mr. Haenszel described what is now known as the Mantel-Haenszel Method for analysis to assess the relationship between exposure to a hazard and disease rates. [2]
Dealing with things that are not there
The Cochran-Mantel-Haenszel method is a technique that generates an estimate of an association between an exposure and an outcome after adjusting for or taking into account confounding. The method is used with a dichotomous outcome variable and a dichotomous risk factor. We stratify the data into two or more levels of the confounding factor (as we did in the example above). In essence, we create a series of two-by-two tables showing the association between the risk factor and outcome at two or more levels of the confounding factor, and we then compute a weighted average of the risk ratios or odds ratios across the strata (i.e., across subgroups or levels of the confounder).[3]
References
Transmissible Party: 1st Anniversary
Yes, Time Flies !
 It is already a year ago that Transmissible was established in the Netherlands. And since June 1, 2016, the little boy has learned to walk.
It is already a year ago that Transmissible was established in the Netherlands. And since June 1, 2016, the little boy has learned to walk.
Do you remember how good it felt, to be on your own two feet for the first time? Exhilarating!
Meanwhile, we have engaged in exciting public health projects, got connected to a growing group of great clients, developed partnerships with inspiring colleagues, and most of all: had fun doing it.
Reasons to be cheerful. And to party obviously 🙂
A day to remember: Lady Mary Wortley Montagu

Lady Montagu in Turkish dress by Jean-Étienne Liotard, ca. 1756, Palace on the Water in Warsaw
It is 328 years ago today, that Lady Mary Wortley Montagu (born Mary Pierrepont) was baptized in London (Nottinghamshire) on 26 May 1689. She was an English aristocrat, letter writer and poet. Lady Mary is today chiefly remembered for her letters, particularly her letters from travels to the Ottoman Empire, as wife to the British ambassador to Turkey, which have been described by Billie Melman as “the very first example of a secular work by a woman about the Muslim Orient”. Aside from her writing, Lady Mary is also known for introducing and advocating for smallpox inoculation to Britain after her return from Turkey. [1]
The Encyclopedia Brittanica describes her as colourful. The daughter of the 5th Earl of Kingston and Lady Mary Fielding (a cousin of the novelist Henry Fielding), she eloped with Edward Wortley Montagu, a Whig member of Parliament, rather than accept a marriage that had been arranged by her father. In 1714 the Whigs came to power, and Edward Wortley Montagu was in 1716 appointed ambassador to Turkey, taking up residence with his wife in Constantinople (now Istanbul). After his recall in 1718, they bought a house in Twickenham, west of London. For reasons not wholly clear, Lady Mary’s relationship with her husband was by this time merely formal and impersonal. [2]
Life in Turkey
In 1716, Edward Wortley Montagu was appointed Ambassador at Istanbul. In August 1716, Lady Mary accompanied him to Vienna, and thence to Adrianople and Istanbul. He was recalled in 1717, but they remained at Istanbul until 1718. While away from England, the Wortley Montagu’s had a daughter on 19 January 1718, who would grow up to be Mary, Countess of Bute. After an unsuccessful delegation between Austria and Turkey/Ottoman Empire, they set sail for England via the Mediterranean, and reached London on 2 October 1718.
The story of this voyage and of her observations of Eastern life is told in Letters from Turkey, a series of lively letters full of graphic descriptions; Letters is often credited as being an inspiration for subsequent female travellers/writers, as well as for much Orientalist art. During her visit she was sincerely charmed by the beauty and hospitality of the Ottoman women she encountered, and she recorded her experiences in a Turkish bath. She also recorded a particularly amusing incident in which a group of Turkish women at a bath in Sofia, horrified by the sight of the stays she was wearing, exclaimed that
“the husbands in England were much worse than in the East, for [they] tied up their wives in little boxes, the shape of their bodies”.
Lady Mary wrote about misconceptions previous travellers, specifically male travellers, had recorded about the religion, traditions and the treatment of women in the Ottoman Empire. Her gender and class status provided her with access to female spaces, that were closed off to males. Her personal interactions with Ottoman women enabled her to provide, in her view, a more accurate account of Turkish women, their dress, habits, traditions, limitations and liberties, at times irrefutably more a critique of the Occident than a praise of the Orient.
Lady Mary returned to the West with knowledge of the Ottoman practice of inoculation against smallpox, known as variolation.
Immigrating the concept of variolation
Lady Mary Wortley Montagu defied convention most memorably by introducing smallpox inoculation to Western medicine after witnessing it during her travels and stay in the Ottoman Empire. In the Ottoman Empire, she visited the women in their segregated zenanas, making friends and learning about Turkish customs. There she witnessed the practice of inoculation against smallpox—variolation—which she called engrafting, and wrote home about it a number of her letters, the most famous being her 1 April 1 1717 “Letter to a Friend”. Variolation used live smallpox virus in the pus taken from a smallpox blister in a mild case of the disease and introduced it into scratched skin of a previously uninfected person to promote immunity to the disease. Lady Mary’s brother had died of smallpox in 1713 and her own famous beauty had been marred by a bout with the disease in 1715.
Lady Mary was eager to spare her children, thus, in March 1718 she had her nearly five-year-old son, Edward, inoculated with the help of Embassy surgeon Charles Maitland. On her return to London, she enthusiastically promoted the procedure, but encountered a great deal of resistance from the medical establishment, because it was an Oriental folk treatment process.
In April 1721, when a smallpox epidemic struck England, she had her daughter inoculated by Maitland, the same physician who had inoculated her son at the Embassy in Turkey, and publicised the event. This was the first such operation done in Britain. She persuaded Princess Caroline to test the treatment. In August 1721, seven prisoners at Newgate Prison awaiting execution were offered the chance to undergo variolation instead of execution: they all survived and were released. [3] Controversy over smallpox inoculation intensified, however, Caroline, Princess of Wales was convinced. The Princess’s two daughters were successfully inoculated in April 1722 by French-born surgeon Claudiius Amyand. In response to the general fear of inoculation, Lady Mary, under a pseudonym, wrote and published an article describing and advocating in favor of inoculation in September 1722.
In later years, Edward Jenner, who was 13 years old when Lady Mary died, developed the much safer technique of vaccination using cowpox instead of smallpox. As vaccination gained acceptance, variolation gradually fell out of favour.
References:
A day to remember: Edward Jenner, father of immunization, was born

Edward Jenner. Pastel by John Raphael Smith.
It was 268 years ago today, on 17 May 1749, that Edward Anthony Jenner was born in Berkeley, Gloucestershire, as the eighth of nine children. His father, the Reverend Stephen Jenner, was the vicar of Berkeley, so Jenner received a strong basic education.
He went to school in Wotton-under-Edge and Cirencester. During this time, he was inoculated for smallpox, no doubt by a method close to the one propagated by Lady Mary Wortley Montagu.
At the age of 14, he was apprenticed for seven years to Daniel Ludlow, a surgeon of Chipping Sodbury, South Gloucestershire, where he gained most of the experience needed to become a surgeon himself. In 1770, Jenner became apprenticed in surgery and anatomy under surgeon John Hunter and others at St George’s Hospital. William Osler records that Hunter gave Jenner William Harvey’s advice, very famous in medical circles (and characteristic of the Age of Enlightenment):
Don’t think; try.
Hunter remained in correspondence with Jenner over natural history and proposed him for the Royal Society. Returning to his native countryside by 1773, Jenner became a successful family doctor and surgeon, practising on dedicated premises at Berkeley. He also became a master mason on 30 December 1802, in Lodge of Faith and Friendship #449.[1]
Like any other doctor of the time, Edward Jenner carried out variolation to protect his patients from smallpox. However, from the early days of his career Edward Jenner had been intrigued by country-lore which said that people who caught cowpox from their cows could not catch smallpox. This and his own experience of variolation as a boy and the risks that accompanied it led him to undertake the most important research of his life. Cowpox is a mild viral infection of cows. It causes a few weeping spots (pocks) on their udders, but little discomfort. Milkmaids occasionally caught cowpox from the cows. Although they felt rather off-colour for a few days and developed a small number of pocks, usually on the hand, the disease did not trouble them.[3]
Working on protection against smallpox
By 1768, English physician John Fewster had realised that prior infection with cowpox rendered a person immune to smallpox.[22] A similar observation had also been made in France by Jacques Antoine Rabaut-Pommier. In the years following 1770, at least five investigators in England and Germany successfully tested a cowpox vaccine in humans against smallpox. For example, Dorset farmer Benjamin Jesty successfully vaccinated and presumably induced immunity with cowpox in his wife and two children during a smallpox epidemic in 1774, but it was not until Jenner’s work that the procedure became widely understood. Jenner may have been aware of Jesty’s procedures and success.
Noting the common observation that milkmaids were generally immune to smallpox, Jenner postulated that the pus in the blisters that milkmaids received from cowpox (a disease similar to smallpox, but much less virulent) protected them from smallpox.[1]
In May 1796 a dairymaid, Sarah Nelmes, consulted Jenner about a rash on her hand. He diagnosed cowpox rather than smallpox and Sarah confirmed that one of her cows, a Gloucester cow called Blossom, had recently had cowpox. Edward Jenner realised that this was his opportunity to test the protective properties of cowpox by giving it to someone who had not yet suffered smallpox.

Hide from the cow named ‘Blossom’
He chose James Phipps, the eight-year old son of his gardener. On 14th May he made a few scratches on one of James’ arms and rubbed into them some material from one of the pocks on Sarah’s hand. A few days later James became mildly ill with cowpox but was well again a week later. So Jenner knew that cowpox could pass from person to person as well as from cow to person. The next step was to test whether the cowpox would now protect James from smallpox. On 1st July Jenner variolated the boy. As Jenner anticipated, and undoubtedly to his great relief, James did not develop smallpox, either on this occasion or on the many subsequent ones when his immunity was tested again.[3] So, in addition to the name of Edward Jenner, we also need to acknowledge the following names for their contribution to this success: Sarah Nelmes (the milkmaid), James Phipps (the child – guinea pig), The gardner Mr Phipps (for allowing this experiment on his son), and Blossom, the Gloucester Cow, for donating the virus. You can still have a look at Blossom, by the way. Her hide is on display at Saint George’s University. [2]
Phipps was the 17th case described in Jenner’s first paper on vaccination. Donald Hopkins has written,
“Jenner’s unique contribution was not that he inoculated a few persons with cowpox, but that he then proved [by subsequent challenges] that they were immune to smallpox. Moreover, he demonstrated that the protective cowpox pus could be effectively inoculated from person to person, not just directly from cattle.
Jenner successfully tested his hypothesis on 23 additional subjects.[1]

Edward Jenner, vaccinating his son.
Convincing establishment and translation into legal acts
Jenner continued his research and reported it to the Royal Society, which did not publish the initial paper. After revisions and further investigations, he published his findings on the 23 cases. Some of his conclusions were correct, some erroneous; modern microbiological and microscopic methods would make his studies easier to reproduce. The medical establishment deliberated at length over his findings before accepting them. Eventually, vaccination was accepted, and in 1840, the British government banned variolation – the use of smallpox to induce immunity – and provided vaccination using cowpox free of charge.[1]
Later life and death
In 1803 in London, he became president of the Jennerian Society, concerned with promoting vaccination to eradicate smallpox. The Jennerian ceased operations in 1809. In 1808, with government aid, the National Vaccine Establishment was founded, but Jenner felt dishonoured by the men selected to run it and resigned his directorship. Jenner became a member of the Medical and Chirurgical Society on its founding in 1805 (now the Royal Society of Medicine) and presented several papers there. Jenner was also elected a foreign honorary member of the American Academy of Arts and Sciences in 1802, and a foreign member of the Royal Swedish Academy of Sciences in 1806. Returning to London in 1811, Jenner observed a significant number of cases of smallpox after vaccination. He found that in these cases the severity of the illness was notably diminished by previous vaccination. In 1821, he was appointed physician extraordinary to King George IV, and was also made mayor of Berkeley and justice of the peace. He continued to investigate natural history, and in 1823, the last year of his life, he presented his “Observations on the Migration of Birds” to the Royal Society.
Jenner was found in a state of apoplexy on 25 January 1823, with his right side paralysed. He never fully recovered and eventually died of an apparent stroke, his second, on 26 January 1823, aged 73. He was buried in the Jenner family vault at the Church of St. Mary’s, Berkeley, Gloucestershire. Jenner was survived by one son and one daughter, his elder son having died of tuberculosis aged 21.[1]
References
A day to remember – Florence Nightingale
 Florence Nightingale, (12 May 1820 – 13 August 1910) was an English social reformer and statistician, and the founder of modern nursing.
Florence Nightingale, (12 May 1820 – 13 August 1910) was an English social reformer and statistician, and the founder of modern nursing.
She came to prominence while serving as a manager of nurses trained by her during the Crimean War, where she organised the tending to wounded soldiers. She gave nursing a highly favourable reputation and became an icon of Victorian culture, especially in the persona of “The Lady with the Lamp” making rounds of wounded soldiers at night.
As a young woman, Nightingale was described as attractive, slender and graceful. While her demeanour was often severe, she was said to be very charming and possess a radiant smile. Her most persistent suitor was the politician and poet Richard Monckton Milnes, but after a nine-year courtship she rejected him, convinced that marriage would interfere with her ability to follow her calling to nursing.
In 1860, Nightingale laid the foundation of professional nursing with the establishment of her nursing school at St Thomas’ Hospital in London. It was the first secular nursing school in the world, now part of King’s College London. In recognition of her pioneering work in nursing, the Nightingale Pledge taken by new nurses, and the Florence Nightingale Medal, the highest international distinction a nurse can achieve, were named in her honour, and the annual International Nurses Day is celebrated around the world on her birthday. Her social reforms include improving healthcare for all sections of British society, advocating better hunger relief in India, helping to abolish prostitution laws that were over-harsh to women, and expanding the acceptable forms of female participation in the workforce.
Nightingale was a prodigious and versatile writer. In her lifetime, much of her published work was concerned with spreading medical knowledge. Some of her tracts were written in simple English so that they could easily be understood by those with poor literary skills. She also helped popularise the graphical presentation of statistical data. Much of her writing, including her extensive work on religion and mysticism, has only been published posthumously.[1]
Statistics and sanitary reform
Florence Nightingale exhibited a gift for mathematics from an early age and excelled in the subject under the tutelage of her father. Indeed, Nightingale is described as “a true pioneer in the graphical representation of statistics”, and is credited with developing a form of the pie chart now known as the polar area diagram, or occasionally the Nightingale rose diagram, equivalent to a modern circular histogram, to illustrate seasonal sources of patient mortality in the military field hospital she managed. Nightingale called a compilation of such diagrams a “coxcomb”, but later that term would frequently be used for the individual diagrams. She made extensive use of coxcombs to present reports on the nature and magnitude of the conditions of medical care in the Crimean War to Members of Parliament and civil servants who would have been unlikely to read or understand traditional statistical reports. In 1859, Nightingale was elected the first female member of the Royal Statistical Society. She later became an honorary member of the American Statistical Association.
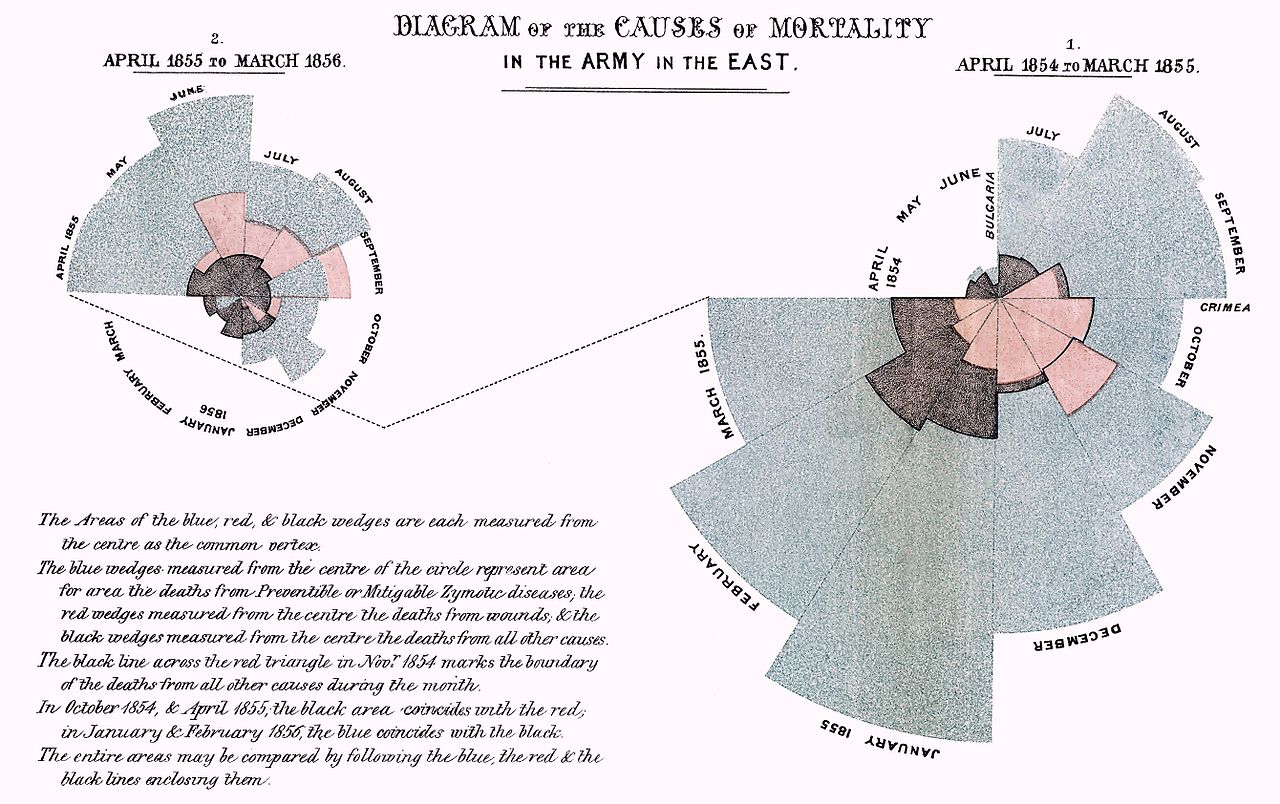
Her attention turned to the health of the British army in India and she demonstrated that bad drainage, contaminated water, overcrowding and poor ventilation were causing the high death rate. She concluded that the health of the army and the people of India had to go hand in hand and so campaigned to improve the sanitary conditions of the country as a whole.
Nightingale made a comprehensive statistical study of sanitation in Indian rural life and was the leading figure in the introduction of improved medical care and public health service in India. In 1858 and 1859, she successfully lobbied for the establishment of a Royal Commission into the Indian situation. Two years later, she provided a report to the commission, which completed its own study in 1863. “After 10 years of sanitary reform, in 1873, Nightingale reported that mortality among the soldiers in India had declined from 69 to 18 per 1,000”.
At the same time she combined with the retired sanitary reformer Edwin Chadwick to persuade Stansfeld to devolve powers to enforce the law to Local Authorities, eliminating central control by medical technocrats. Her Crimean War statistics had convinced her that non-medical approaches were more effective given the state of knowledge at the time. Historians now believe that both drainage and devolved enforcement played a crucial role in increasing average national life expectancy by 20 years between 1871 and the mid-1930s during which time medical science made no impact on the most fatal epidemic diseases.
Artwork
If you want to enjoy artwork of Florence Nightingale, then feel welcome to visit the Facebook page of Saint Roch’s Corner, and browse through the album ‘Nurses at Work‘.
References
Global Hand Hygiene Day
5 May is global hand hygiene day. Each year the “SAVE LIVES: Clean Your Hands” campaign by the World Health Organisation, aims to progress the goal of maintaining a global profile on the importance of hand hygiene in health care and to ‘bring people together’ in support of hand hygiene improvement globally.
This year the theme is “Fight antibiotic resistance – it’s in your hands”, with calls to action for different target groups:
- Health workers: “Clean your hands at the right times and stop the spread of antibiotic resistance.”
- Hospital Chief Executive Officers and Administrators: “Lead a year-round infection prevention and control programme to protect your patients from resistant infections.”
- Policy makers: “Stop antibiotic resistance spread by making infection prevention and hand hygiene a national policy priority.”
- IPC leaders: “Implement WHO’s Core Components for infection prevention, including hand hygiene, to combat antibiotic resistance.”
There is also a great link to a wealth of online training materials, in particular on hygiene in healthcare settings.
One of the challenges within a health care organisation is to have all health professionals on the same page regarding the hand hygiene. Sharing best practice workshops help to get professionals on the same page and agree on joint approaches. Transmissible helps you raise awareness with audiovisual messages and serious games. In addition, we help you organise and moderate workshops.
Do you want to see an overview of 250 years of Hand Hygiene? Click on the timeline below
[h5p id=”7″]A Day to Remember: Carl Friedrich Gauss
 Johann Carl Friedrich Gauss (30 April 1777 Braunschweig – 23 February 1855 Göttingen) was a German mathematician who contributed significantly to many fields, including number theory, algebra, statistics, analysis, differential geometry, geodesy, geophysics, mechanics, electrostatics, astronomy, matrix theory, and optics.
Johann Carl Friedrich Gauss (30 April 1777 Braunschweig – 23 February 1855 Göttingen) was a German mathematician who contributed significantly to many fields, including number theory, algebra, statistics, analysis, differential geometry, geodesy, geophysics, mechanics, electrostatics, astronomy, matrix theory, and optics.
So, what does he have to do with Public Health?
Among the many scientific fields that Gauss has made major contributions to, we focus here on his contribution to statistics and estimates. Before describing that part of his work, let’s first take a look at his early years.
Johann Carl Friedrich Gauss was born in Brunswick (Braunschweig), in the Duchy of Brunswick-Wolfenbüttel (now part of Lower Saxony, Germany), as the son of poor working-class parents. His mother was illiterate and never recorded the date of his birth, remembering only that he had been born on a Wednesday, eight days before the Feast of the Ascension, which itself occurs 39 days after Easter. Gauss later solved this puzzle about his birthdate in the context of finding the date of Easter, deriving methods to compute the date in both past and future years.
Gauss was a child prodigy. A contested story relates that, when he was eight, he figured out how to add up all the numbers from 1 to 100. There are many other anecdotes about his precocity while a toddler, and he made his first ground-breaking mathematical discoveries while still a teenager. He completed Disquisitiones Arithmeticae, his magnum opus, in 1798 at the age of 21, though it was not published until 1801. This work was fundamental in consolidating number theory as a discipline and has shaped the field to the present day. Gauss’s intellectual abilities attracted the attention of the Duke of Brunswick, who sent him to the Collegium Carolinum (now Braunschweig University of Technology), which he attended from 1792 to 1795, and to the University of Göttingen from 1795 to 1798. While at university, Gauss independently rediscovered several important theorems.
Contribution to Statistics
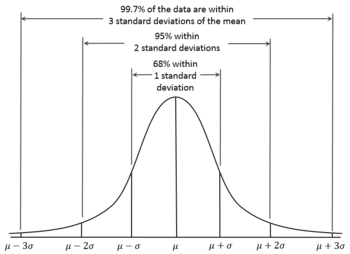 The name ‘Gauss’ could bring back memories of our high-school mathematics lessons about ‘normal distributions’. In 1809, Gauss developed the formula for the normal distribution and showed that errors were fit well by this distribution. In general, Gauss’s contributions to statistics may be classed under the general heading of least squares, although this gives little indication of their scope or impact. His first exposition of least squares was given in 1809 when he was 31, but these ideas must have been formulated much earlier, apparently first when he was 17. In Book 2, Section 3 of his book on planetary orbits, he discussed the estimation of the six constants or parameters that determine the elliptical orbit on the basis of n > 6 observations. He began it in article 175 with:
The name ‘Gauss’ could bring back memories of our high-school mathematics lessons about ‘normal distributions’. In 1809, Gauss developed the formula for the normal distribution and showed that errors were fit well by this distribution. In general, Gauss’s contributions to statistics may be classed under the general heading of least squares, although this gives little indication of their scope or impact. His first exposition of least squares was given in 1809 when he was 31, but these ideas must have been formulated much earlier, apparently first when he was 17. In Book 2, Section 3 of his book on planetary orbits, he discussed the estimation of the six constants or parameters that determine the elliptical orbit on the basis of n > 6 observations. He began it in article 175 with:
“To this end let us leave our special problem, and enter upon a very general discussion and one of the most fruitful in every application of the calculus to natural philosophy”.
His second exposition was presented in a series of three lengthy papers to the Royal Society of Göttingen. Here he introduced the subject as follows
“The problem is certainly the most important which the application of mathematics to natural philosophy presents”.
In spite of the importance he obviously attributed to the subject, as evidenced by the above quotations, he appeared not to have returned to it in later years, at least in print, although he continued lecturing on it.
It is important to note here that Adrien-Marie Legendre published the first description of the metho, in 1805, and was responsible for the name “least squares”. However, it was Gauss, not Legendre, who developed the method into a statistical tool, embedding it into a statistical framework, involving the probabilistic treatment of observational errors, and thus set the famous linear model on its modern course. For example, consider the following quotations from Fisher:
“Gauss, moreover, approached the problem of statistical estimation in an empirical spirit, raising the
question of the estimation not only of probabilities, but of other quantitative parameters. He perceived the aptness for this purpose of the Method of Maximum Likelihood, although he attempted to derive and justify this method from the principle of inverse probability… Gauss, further, perfected the systematic fitting of regression formulae, simple and multiple, by the method of least squares…”
OK, but what’s the use for public health?
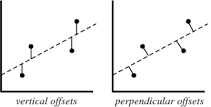 The least squares method is a form of mathematical regression analysis that finds the line of best fit for a dataset, providing a visual demonstration of the relationship between the data points. Public Health relies heavily on measurements of diseases and their determinants within populations. Such measurements are often prone to various degrees of error. Public Health Scientists use mathematics to describe relationships between their measurements of determinants (risk factors, protective factors) and their measurements of disease. The Least Squares Method helps them to find the best fitting relation (usually a line) in their data. Gauss has given us a tool to describe those relations, which helps us to understand how factors influence the risk of disease.
The least squares method is a form of mathematical regression analysis that finds the line of best fit for a dataset, providing a visual demonstration of the relationship between the data points. Public Health relies heavily on measurements of diseases and their determinants within populations. Such measurements are often prone to various degrees of error. Public Health Scientists use mathematics to describe relationships between their measurements of determinants (risk factors, protective factors) and their measurements of disease. The Least Squares Method helps them to find the best fitting relation (usually a line) in their data. Gauss has given us a tool to describe those relations, which helps us to understand how factors influence the risk of disease.
References:
- Carl Friedrich Gauss, in: Wikipedia. Accessed 29 April 2017
- Historia Mathematica 5 (1978), 183-203 Gauss’s Contributions To Statistics By D, A, Sprott.
- Fisher, 1973 Statistical Methods and Scientific Inference, New York (Hafner Press)
Immunisation Week 2017
The World Immunisation Week 2017 has started on April 24. This global event aims to raise awareness about the critical importance of full immunisations throughout life.
Follow #VaccinesWork on Twitter, to experience all that happens around this global health event
Immunization saves millions of lives and is widely recognized as one of the world’s most successful and cost-effective health interventions. Today, there are still 19.4 million unvaccinated and under-vaccinated children in the world.
Despite improvements in individual countries and a strong global rate of new vaccine introduction, all of the targets for disease elimination—including measles, rubella, and maternal and neonatal tetanus—are behind schedule. In order for everyone, everywhere to survive and thrive, countries must make more concerted efforts to reach GVAP goals by 2020. Additionally, those countries that have achieved or made forward progress towards achieving the goals must work to sustain those efforts over time.
Why immunization matters now more than ever
Expanding access to immunization is crucial to achieving the Sustainable Development Goals. Routine immunization is a building block of strong primary health care and universal health coverage—it provides a point of contact for health care at the beginning of life and offers every child the chance at a healthy life from the start.
Immunization is also a fundamental strategy in achieving other health priorities, from controlling viral hepatitis, to curbing antimicrobial resistance, to providing a platform for adolescent health and improving antenatal and newborn care.

Debating Trust in Vaccines
 The Biovision Life Sciences Forum offers an interactive platform bringing together actors in research, innovation, partnerships and finance to debate existing and future health issues and how to address them. One of those topics is Trust in Vaccines: a Workshop on Thursday morning 6 April 2017, discussing three questions:
The Biovision Life Sciences Forum offers an interactive platform bringing together actors in research, innovation, partnerships and finance to debate existing and future health issues and how to address them. One of those topics is Trust in Vaccines: a Workshop on Thursday morning 6 April 2017, discussing three questions:
- How to reach a better understanding of vaccines? What is needed?
- What do we expect from new vaccines?
- What would improve vaccine coverage?
Actors from industry, the public sector, educators, academia, and communicators, discussed these questions, making the link to the concept of trust. Trust was said to require a foundation of knowledge as well as belief. Factors influencing knowledge touch on education and communication. On the one hand, there has never in history been such access to knowledge, yet increasingly we face false facts, and the challenge to identify truth from lie. In addition, belief is not necessarily based on knowledge and facts, yet may be more a personal choice based on views and social context.
A 1-hour debate session for a larger audience followed the workshop. Transmissible provided the debate moderation among four expert speakers on the main stage, including Dr Alain Fischer (Institute Imagine), Dr Marie-Paule Kieny (WHO), Dr David Loew (Sanofi), and Dr Cyril Schiever (MSD). Speakers agreed on the need to invest more in education and communication around vaccines (towards the public and professionals), as well as improving access to vaccines and research. Issues of debate included (legal) compulsory vaccination (may in some countries actually feed into vaccine rejection), incentives for health professionals (risks perception that doctors have financial motives for advising vaccines) and public shaming of decisions to refuse vaccines (may create social polarization, bringing us further from a shared trust).
The speakers shared awareness that the individual citizen expects more and more autonomy to decide (wants to be in charge) and wants to be well informed. This changes the classic doctor-patient relationship fundamentally. We will need to find solutions to deal with that new reality. In addition, the generation of doctors who have seen first-hand the burden of vaccine-preventable diseases will retire, and millennial doctors, who ‘live online’, need to be made aware of what these diseases mean in reality. Will immersive simulation games be a helpful tool in such education? Investing in research for online platforms, sharing open access health data, infection modelling and simulation tools may prove relevant to explore.
Before the debate started, Arnold Bosman posed a provocative statement: the age of experts has ended, and the age of the self-empowered citizen has begun:
The debate session can be viewed here:
https://www.youtube.com/embed/0zbCWgklnw4
A day to remember: Pierre-Simon de Laplace
 Pierre-Simon, marquis de Laplace (23 March 1749 – 5 March 1827) was an influential French scholar whose work was important to the development of mathematics, statistics, physics and astronomy. He summarized and extended the work of his predecessors in his five-volume Mécanique Céleste (Celestial Mechanics) (1799–1825). This work translated the geometric study of classical mechanics to one based on calculus, opening up a broader range of problems. In statistics, the Bayesian interpretation of probability was developed mainly by Laplace.
Pierre-Simon, marquis de Laplace (23 March 1749 – 5 March 1827) was an influential French scholar whose work was important to the development of mathematics, statistics, physics and astronomy. He summarized and extended the work of his predecessors in his five-volume Mécanique Céleste (Celestial Mechanics) (1799–1825). This work translated the geometric study of classical mechanics to one based on calculus, opening up a broader range of problems. In statistics, the Bayesian interpretation of probability was developed mainly by Laplace.
Laplace is remembered as one of the greatest scientists of all time. Sometimes referred to as the French Newton or Newton of France, he has been described as possessing a phenomenal natural mathematical faculty superior to that of any of his contemporaries.
In 1812, Laplace issued his Théorie analytique des probabilités in which he laid down many fundamental results in statistics. The first half of this treatise was concerned with probability methods and problems, the second half with statistical methods and applications. Laplace’s proofs are not always rigorous according to the standards of a later day, and his perspective slides back and forth between the Bayesian and non-Bayesian views with an ease that makes some of his investigations difficult to follow, but his conclusions remain basically sound even in those few situations where his analysis goes astray. In 1819, he published a popular account of his work on probability.[1]
The theory of probability, in essence, is nothing more than common sense, reduced to a calculation.
Pierre Laplace realized that certain error was inherent in all calculations. Instead of ignoring the error, he chose to quantify it, and the field of statistics was born. He even demonstrated that there was a mathematical distribution to the likelihood of error observed in given experiments. His student, Karl Peason, then took Laplace one step further and showed that not only there is a probability to the likelihood of error, but even our own measurements are probabilities. Pearson’s revolutionary work laid the basis for modern statistics. After that, the early twentieth century geneticist Ronald Fisher introduced randomization and p-values, followed by A.Bradford-Hill, who applied there concepts to medical illnesses and founded clinical epidemiology.[2]

Laplace died in Paris in 1827. His brain was removed by his physician, François Magendie, and kept for many years, eventually being displayed in a roving anatomical museum in Britain. It was reportedly smaller than the average brain. Laplace was buried at Père Lachaise in Paris but in 1888 his remains were moved to Saint Julien de Mailloc in the canton of Orbec and reinterred on the family estate. [1]
References:
- Pierre-Simon Laplace, in Wikipedia, accessed on 22 March 2017.
- A Clinician’s Guide to Statistics and Epidemiology in Mental Health. By S. Nassir Ghaemi
Summer Schools for Public Health in Europe, 2017 – a selection
Each summer there are plenty of opportunities for continuous professional education in public health. This post presents a selection, an overview of what we could find on the web quickly. If you know of a Summer School on Public Health Topics that is not on this list, please post the information below.
The Observatory Summer School
http://theobservatorysummerschool.org/edition-2017/
Sunday 23 July – Saturday 29 July 2017
The course takes place on the island of San Servolo, one of the most beautiful and awe-inspiring islands of the Venice lagoon.
The six‐day course includes formal teaching but has at its core the experiences of participants in practice. A highly participative approach emphasises group work that cuts across themes, participant presentations, round tables and panel discussions. It mobilises the latest evidence and a multidisciplinary team of experts with a track record in the analysis, implementation and evaluation of person‐centred health systems. Course participants will also be able to share perspectives with and gain insights from key international organisations including the World Health Organization, the European Commission and relevant professional, governmental and civil society organisations and to engage in political dialogue with senior policy makers. They will be part of the Summer School tradition, which fosters evidence‐based policy‐making and encourages European health policy debate by raising key issues, sharing learning and building lasting networks.
Annual ECDC Summer School
30 May to 2 June in Stockholm, Sweden.
The goal of the ECDC Summer School is to strengthen the mentoring and technical skills of both ECDC experts and experts within ECDC networks, such as fellowships’ supervisors, by providing an opportunity for networking and scientific exchange on methods for communicable disease prevention and control.
The ECDC invites experts form the EU networks (e.g. Fellowship Training Supervisors) to participate to the Summer School. The school is usually closed for external participants.
Diagnostic data for dummies: The untapped potential of data re-use, Rotterdam, the Netherlands
https://rotterdamsummerschool.com/
9 July 2017 – 12 July 2017
Health care professionals are building up data collections as part of their practice. Smart re-use of these data supports diagnosis and treatment of patients. These data could also be a basis for research and prevention. Furthermore, pooling data with collegues can take health care and prevention to a next level. This summer course provides you with practical knowledge and skills to get the best out of your data sets. You will learn to use state-of-the-art data analysis and visualization tools, while issues such as security, privacy and ethics will be addressed.
Future of Public Health in the Post-2015 World, Finland Kuopio
https://www.uef.fi/en/web/summerschool
14-24 August 2017
This Summer School will cover the following topics:
- Tracking the Sustainable development goals
- Future of non-communicable diseases
- Ageing populations and public health
- Public health nutrition
- Public health in humanitarian crises
- Contemporary environmental aspects of public health
- Sociocultural aspects of public health, health inequalities
- Population health promotion: examples from real life
- Health care systems as sites for public health progress
- Applying epidemiology for public health problem solving
Population and public health, London, UK
https://www.ucl.ac.uk/prospective-students/study-abroad-ucl/summer-school
3-21 July 2017
This module will provide an introduction to definitions used in population and public health, basic theories, and conceptual frameworks linking major determinants of health with a range of individual and population health outcomes.
It will provide an introduction to the history of population health. The role of London in public health research will be explored and the basic measurements of outcomes and risk factors used in public health, and data sources used in population health, will be introduced.
Monitoring and Evaluation of Public Health Programs: Systems Approaches and Techniques, Italy
12 June 2017 – 17 June 2017
This course introduces methods and tools necessary for monitoring and evaluating public health programs during routine public health activities as well as during large scale emergencies and public health crisis. The course will use the case based teaching method developed by the Harvard Business School and examples from the ebola outbreak, recent water crisis and other types of events to describe how evaluation methods can be used to inform public health decision making. The range of topics includes: evaluation planning, survey development and validation techniques, assessment of modern and rapid testing methods; an overview of various methodologies and designs for estimating coverage and changes for a region; methods for evaluating sub-regional performance (i.e. the health districts of a region); and comprehensive monitoring and evaluation approaches that allow for both local and regional assessment. Emphasis will be on the practical aspects of design, analysis and presentation. Students will use a public health systems approach to the evaluation of the programs and discuss as a group the consequences of the decisions they make on the implementation and evaluation of specific public health programs.
Public Health, UK, London
http://www.kcl.ac.uk/study/summer/index.aspx
17 July – 4 August 2017
The module will introduce you to public health; an exciting and growing field which is underpinned by a diverse group of disciplines reflecting both the arts and sciences, employing a range of strategies to promote and protect health and well-being. We will look at the evolution of public health specifically in the UK, but also consider how changing global landscape means that boundaries between populations are increasingly less well demarcated. We will explore the key concepts of epidemiology and the broader determinants of health. The complexity around evidence-based practice, policy and politics will also be considered. This module will provide a good foundation for anybody thinking about embarking upon a career in which public health can play a role.
Environmental epidemiology, Utrecht, the Netherlands
https://www.utrechtsummerschool.nl/
19-30 June 2017
The objective of the course is to provide the student with insight in the principles and important issues of environmental and occupational epidemiology. Topics that are covered include time series analyses, assessment of dose-response relationships, use of geographic information systems in exposure assessment, retrospective cohort studies, ecological studies, (correction for) measurement error in exposure and interpretation of studies on mortality (life expectancy versus body counts). Theory will be illustrated by current and emerging topics like climate change and electromagnetic fields.
Screening and epidemiology, Copenhagen, Denmark
14-22 August 2017
Students will be trained in critical assessment of the evidence on (cancer) screening. The course aims to provide a solid introduction to the major concepts, theories and debates relevant to screening, with particular emphasis on early detection of cancer. Emphasis will be placed on understanding the rationale for screening and evaluation of screening outcomes. Finally, the course will provide an overview of the main cancer screening programs that have been implemented in Denmark.
Research methods in health: Biostatistics, Italy
12 June 2017 – 17 June 2017
This course is designed to provide the student with an understanding of the foundations of biostatistics and of the various statistical techniques that have been developed to answer research questions in the health sciences. Students will be introduced to methods for the comparison of outcome between two groups (t-test and non parametric tests), as well as the extension to the comparison of outcome across several groups (ANOVA); methods for the study of association between two continuous variables (correlation and linear regression); the analysis of contingency tables; the study of survival (time-to-event) data. The afternoon sessions are devoted to discussion and learning to use Stata® to implement materials covered in the morning lectures.
Research methods in health: Epidemiology, Italy
12 June 2017 – 17 June 2017
This course will explore in greater depth the fundamental epidemiologic concepts introduced in Principles of Epidemiology (Week 1). The course will be taught with an emphasis on causal inference in epidemiologic research. Topics will mainly focus on chronic disease epidemiology, with a special emphasis on practical study design. Epidemiologic examples from major chronic diseases/conditions (e.g. heart disease and cancer) will be discussed. Students will revisit the issues of confounding, selection bias, effect modification, and generalizability in the context of these topics. Lectures will be augmented by workshops to illustrate practical examples in the epidemiologic literature. The material covered in Principles of Epidemiology will be assumed of the students entering this course.
Causal Inference in Epidemiology, Italy
5 June 2017 – 10 June 2017
Causal inference from observational data is a key task of biostatistics and of allied sciences such as sociology, econometrics, behavioral sciences, demography, economics, health services research, etc. These disciplines share a methodological framework for causal inference that has been developed over the last decades. This course presents this unifying causal theory and shows how biostatistical concepts and methods can be understood within this general framework. The course emphasizes conceptualization but also introduces statistical models and methods for causal effect estimation. Specifically, this course strives to a) formally define causal concepts such as causal effect and confounding using potential outcomes and counterfactuals, b) identify the conditions required to estimate causal effects using Directed Acyclic Graphs (DAGs), and c) introduce analytical methods that, under those conditions, provide estimates that can be endowed with a causal interpretation. Examples of such methods are regression adjustment, standardization and inverse probability weighting.
Effectiveness Research with Longitudinal Healthcare Databases, Italy
5 June 2017 – 10 June 2017
Large longitudinal healthcare databases have become important tools for studying the utilization patterns and clinical effectiveness of medical products and interventions in a wide variety of care settings. This course will prepare students to identify and use longitudinal databases for their own research. Strengths and limitations of large longitudinal healthcare databases that are commonly used for research will be considered. Special attention will be devoted to nationally representative databases that are critical for comparative effectiveness research. The course focuses on analytic principles and their application to database research. Participants will learn through lectures by experienced faculty and by evaluating published database studies. In computer labs they will learn to implement a database study comparing two medical products in a large healthcare claims database. The project will be conducted using the Aetion platform with an intuitive user interface that does not require any programming skills. The course requires a working understanding of epidemiologic study designs and typical analysis strategies. The target audience consists of researchers working in academia, medical product industry, health plans, government institutions, regulatory agencies, who have access to large longitudinal healthcare databases. They may use such data to evaluate the effectiveness of medical interventions and care patterns, to understand the comparative effectiveness and safety of medical products (drugs, devices), to test the impact of coverage policy changes, or to monitor the outcome of risk-sharing arrangements.
European Public Health in a Globalising World – introducing policy, research and practice from a European healthcare, disease prevention and health promotion perspective, Maastricht, the Netherlands.
https://www.maastrichtuniversity.nl/maastricht-summer-school-2017-courses-tuition-fees
(3-21 July)
The course provides an overview of modern health challenges in Europe and how they are embraced by a variety of stakeholders: policy makers, researchers, practitioners and the
civil society. The course focus on three perspectives: Firstly, health in Europe, hence, what is the health status across the European countries, how do the health systems look like, what is major challenges for individual countries. Secondly, the perspective of European health which focuses on integration and collaboration among Member States within the European Union (EU) and more widely according to the WHO European region. Lastly, European health in a globalised world is assessed. The course combines theory with practice through lectures, tutorials and field visits.
This course consists of 32 class hours divided over 3 weeks. Students earn 6 ECTS credits when they obtain a passing grade.
Creating health literate societies: bridging the gap of inequality, Maastricht, the Netherlands
https://www.maastrichtuniversity.nl/maastricht-summer-school-2017-courses-tuition-fees
(24 July – 11 August)
Health literacy is an important asset for managing health in the 21st century. Health literacy entails the knowledge, motivation, and competency to access, understand, appraise and apply information to make decisions concerning healthcare, disease prevention and health promotion in everyday life to improve quality of life during the life course. People-centred care and co-production of health are new avenues for the future, which demands active citizens and responsive systems. However, recent research has estimated that almost one have limited health literacy and that under-served population groups such as the elderly, less educated people and groups with low socio-economic status are even worse off. Strategic action is needed to bridge the gap.
The aim of the course is to equip students with health literacy leadership skills to play an active role as professionals in creating health literate societies. Students will explore the concept and its application in research, policy and practice. They will gain competency in developing health literacy strategies and programmes in high, middle and low-income countries to bridge the global gap of health inequality. Through the problem-based learning method they will gain knowledge on how to measure health literacy, how to design complex health literacy interventions and how to build health literacy friendly organisations, communities, cities and societies.
This course consists of 32 class hours divided over 2-3 weeks. Students earn 6 ECTS credits when they obtain a passing grade.
Public Health Assets: Mapping and Mobilizing Health Assets, Alicante, Spain
https://etcsummerschool.files.wordpress.com/2017/01/programme_2017.pdf
17-28 July 2017
Organised by the European Training Consortium in Public Health and Health Promotion (ETC-PHHP), this course is held in Alicante, Spain. Central in this 26th ETC Summer School is the perspective of Public Health Assets complementary to the classic needs/deficit approach used in the majority of public health and health promotion programmes. The notion of assets has its origin in Asset Based Community Development (ABCD) and is nowadays often applied as a public health intervention tool. One special aim of this course will be discussing possible connections between Salutogenesis developed by Aaron Antonovsky and the Assets model of health. The aim of the residential Summer School in Alicante is to provide an international and multidisciplinary forum for the exchange of knowledge and skills and opportunities to explore:
- People-environment interaction in relation to the promotion of health and wellbeing (health promotion)
- Internal and external resources and mechanisms that enable people to participate fully in society
- System approaches to create synergy between Salutogenesis and Public Health Assets
The 2-week course opens with a 1-day International Concha Colomer Symposium which is open to a wider audience of local and international professionals and academics with an interest in health promotion or the theme ‘Public Health Assets’. Both theoretical and practical elements of health promotion are included throughout the programme, which is underpinned by an emphasis on participant interaction. This includes field visits to exemplary local programmes and opportunities for sharing knowledge and experiences with international colleagues involved in promoting health in its broadest sense. All participants are involved in preparing a “country presentation” to present to all participants during the beginning of the course and will later engage in developing a project (including a research component) as part of an international multidisciplinary working group.
Meta-Analysis in Systematic Reviews for Food And Feed Related Topics: York, United Kingdom
1-2 June 2017
The aim of this course, facilitated by the York Health Economics Consortium (YHEC), is to provide a comprehensive introduction to meta-analysis for systematic reviews of food and feed related topics. Participants will use software to conduct pairwise meta-analysis and meta-regression.
Systematic Reviews and Meta-Analysis: Bristol, United Kingdom
http://training.cochrane.org/systematic-reviews-and-meta-analysis-bristol-united-kingdom
12-15 June 2017
This course is designed for clinicians, researchers, public health specialists and other health care professionals who want to perform and/or critically appraise systematic reviews and meta-analyses. The course predominantly covers systematic reviews of healthcare interventions, with some material being equally relevant to systematic reviews of other topics. Towards the end of the course, special sessions examine issues in systematic reviews and meta-analyses of observational studies and diagnostic test accuracy.
3rd European Summer School in Evidence-Based Public Health, Munich, Germany
4-8 July 2017
http://www.evidencebasedpublichealth.co.uk/
Evidence-based public health is the development, implementation and evaluation of effective programmes and policies in public health through application of principles of scientific reasoning, including systematic uses of data and information systems. This idea, inspired by the concepts of evidence-based medicine is gaining momentum – nationally, as well as internationally.
Following the success of the second European Summer School in Evidence Based Public the third event will return to the Pettenkofer School of Public Health, Ludwig-Maximilians-Universitat, Munich and will be held on July 4th to 8th 2016.
International Summer School – Health Policy & Management, University of Bayreuth, Germany
July 2017
https://www.daad.de/deutschland/studienangebote/international-programs/en/?p=d&s=sk&id=1113
The course Health Policy & Management looks at comparative politics and policy in high-income countries, focusing on European Union member states and North America. It will focus both on the relationship between political and policy-making structures and on health systems and policies. The course will start with basic structural variables and questions about their contemporary relevance: social insurance and national health service systems as well as forms of centralisation and decentralisation in politics. It will then cover the ways in which these basic structures shape conflicts over priorities, structures, services and equity in different policy areas, namely, health care financing reform, primary health care, hospital care, chronic and integrated care, and public health care (prevention and communicable disease control). The basic logic is then applied and extended to the globalisation and Europeanisation of health care by discussing the ways in which the EU does or does not shape European health systems and the impact and regulation of global markets in the context of services, professionals, and patients.
Summer School – “Social Cohesion: Concept, Implementation and Impact Evaluation”, Kyrgyzstan
11–18 June 2017
SIPRI and the University of Central Asia (UCA) are pleased to invite applicants to a summer school on social cohesion in Kyrgyzstan.
Social cohesion has emerged over the last two decades as an important concept in both academic and political discourse. Quality of connections and cooperation between people and groups is essential to stability and development. Social cohesion—convergence across groups in society—provides a structure that helps ensure greater degrees of predictability and certainty in inter- and intra-group relations. While there is no guarantee that all groups within a society will agree on all issues, convergence across groups offers an incentive for groups to coexist as a peaceful society.
The summer school ‘Social Cohesion: Concept, Implementation and Impact Evaluation’ is a capacity-building activity for researchers, practitioners and policymakers in Kyrgyzstan and Central Asia. It is organized as part of the ‘Social Cohesion through Community-based Development’ project implemented in Kyrgyzstan from 2014 to 2017 and funded by the World Bank and the Aga Khan Foundation. The project aims to identify, pilot and build capacity for social cohesion mechanisms in community-driven development approaches.
6th international BfR-Summer Academy on Risk Assessment and Risk Communication, Berlin, Germany
http://www.bfr.bund.de/cm/349/6-th-bfr-summer-academy-flyer.pdf
3 – 14 July 2017
The international BfR-Summer Academy on Risk Assessment and Risk Communication in the area of Food safety will be conducted by highly qualified and experienced BfR-scientists as well as external experts with profound and long standing experience in risk assessment of chemical or microbial risks. The BfR-Summer Academy is intended for the members of staff of your organisations engaged in food and feed safety, who have already experience in risk assessment analysis. The participants will acquire practical risk assessment analysis and gain deeper understanding in risk communication measures. Consequently this BfR-Summer Academy is from professionals to professionals.
European Drugs Summer School, Lisbon, Portugal (No, it is probably not what you think…)
http://www.drugsummerschool.cies.iscte-iul.pt/np4/23/
26 June-7 July 2017
This two-week summer school prepares professionals and students to meet the complex policy challenges that face Europe in the field of drugs. Involving scientific experts from the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), but also university professors and policy-makers, it provides a multi-disciplinary and inclusive approach to the study of the drug problem in Europe and beyond. As this summer school will give 6 ECTS credits for its courses, students can easily transfer credits to other European universities using the ECTS system. Credits will be attributed formally to post graduate students (Master and PHD), but it is possible to allow undergraduate to use these credits in their home universities.
Summer Courses Global Health, Copenhagen, Denmark
http://globalhealth.ku.dk/studies/summer_courses/
Various courses, all summer long. Deadline 1 April. Read fast and be quick!
The University of Copenhagen’s International Summer Programme offers a number of popular global health-related summer courses, which are open to both students and external applicants with an interest in global health issues.
The 250-year-old history of hand hygiene in healthcare
Early days: between recognition and ridicule
Alexander Gordon documented the first systematic observation regarding hand hygiene more than 200 years ago in Aberdeen, Scotland. He recognized the contagiousness of the puerperal fever and the role of caretakers’ hands in cross-transmission leading to outbreaks and high fatality. Over the centuries, several pioneers suggested the hand hygiene as a preventive measure, with various degrees of professional recognition. Work of Ignaz Phillip Semmelweis, Hungarian obstetrician who demonstrated experimentally that appropriate hand hygiene significantly reduced risk of puerperal infections and maternal deaths, was rejected by peers. Similarly, Gordon’s theory was ridiculed and dismissed by the doctors, midwives and the public of Aberdeen. And even when Watson and Holmes received recognition for their medical achievements, they had to endure attacks on their theories of hygiene, often finding their recommendations disregarded.
Evidence base helps, yet attitude is key
After 1870, the germ-theory offered a strong support for earlier hygiene theories, established through discoveries of Pasteur, Koch, and Lister. Yet, even in 21st century compliance to hand hygiene remains low. Current work by Didier Pittet highlights the importance of a multimodal approach. Knowledge of the evidence base behind hygiene alone is not sufficient. Attitude is another important factor, among health care workers. And finally, the system must be designed to allow opportunity for applying hygienic practices. According to Pittet, strategies to improve hand hygiene compliance must include system change, staff education and motivation, the use of performance indicators, and hospital management support.
This poster presents the chronology of these milestones in hand hygiene. It was presented at the Semmelweis Conference in Budapest, 7-8 March 2017.
Authors: Rita Szabo, Andrea Kurcz, Arnold Bosman
A day to remember: August von Wassermann

August von Wassermann (1866)
It was 151 years ago today…..
August Paul von Wassermann (21 February 1866 – 16 March 1925) was a German bacteriologist and hygienist.
Born in Bamberg, with Jewish origins, he studied at several universities throughout Germany, receiving his medical doctorate in 1888 from the University of Strassburg. [1] On September 1, 1891, he entered the newly established Institute for Infectious Diseases, headed by Koch, as an unpaid assistant in both the scientific and clinical divisions, working under Bernhard Proskauer. In February 1893 he became a temporary assistant assigned to problems related to cholera, and from February 1895 to June 1896 he was inspecting physician at the Institute’s antitoxin control station for diphteria, which was transferred in 1896 to the Institute for Serum Research and Testing in the Berlin suburb of Steglitz. Wassermann then returned to the institute itself as an unpaid assistant.
Some felt that Wassermann carried on his investigations as a hobby, and he certainly did not need to earn a living. He was small in stature but, contrary to assertion, he was not stooped or hunch-backed, and he had bright blue (not dark) eyes. He always dressed with extreme elegance.
He was an impulsive man and a superb speaker, expressing his conclusions openly without scientific inhibitions. It was said that his theoretical knowledge was rather weak and that his practical application was not at all that good either, but that he relied on a number of technicians to do his biddings in the laboratory. These were dubbed his scientific coolies. He had an outstanding capacity to render complicated theoretical problems comprehensible to the uninitiated. [2]
In 1906 he became director of the division for experimental therapy and serum research at the institute, followed by a directorship of the department of experimental therapy at the Kaiser-Wilhelm-Gesellschaft for the Advancement of Science in Berlin-Dahlem (1913).
Wassermann developed a complement fixation test for the diagnosis of syphilis in 1906, just one year after the causative organism, Spirochaeta pallida, had been identified by Fritz Schaudinn and Erich Hoffmann. The so-called “Wassermann test” allowed for early detection of the disease (despite its nonspecific symptoms), and thus prevention of transmission. He attributed the development of the test to earlier findings of Jules Bordet and Octave Gengou (complement fixation reaction) and to a hypothesis introduced by Paul Ehrlich in his interpretation of antibody formation.
The Wassermann test remains a staple of syphilis detection and prevention in some areas, although it has often been replaced by more modern alternatives. With Wilhelm Kolle, he published the six-volume Handbuch der Pathogenen Mikroorganismen (Handbook of Pathogenic Microorganisms). [1]
With Rudolf Kraus (1868-1932), Wassermann was co-founder of the Free Association for Microbiology, and he served as president of the Academy for knowledge of Judaism. Although occasionally sarcastic, he was always helpful and kind, even under different conditions. He once characterized himself as a “laboratory worker.” His many honours included orders and decorations from Prussia, Belgium, Japan, Romania, Spain, and Turkey.
In 1921 Wassermann was the first recipient of the Aronson Foundation Prize. However, he was never appointed to a chair at the prestigious Berlin faculty, and was never awarded the Nobel Prize, to which he is said to have been a candidate. In 1895 he married Alice von Taussig of Vienna; they had two sons. [2]
References
A day to remember: Viktor Zhdanov

Viktor Zhdanov, 1958
It was 103 years ago today:
Viktor Mikhailovich Zhdanov was born in the village of Shtepino (Ukraine). Zhdanov (13 February 1914 – 1987) was a virologist and instrumental in the effort to eradicate smallpox globally.
After graduating from Kharkiv Medical Institute in 1936, he spent the next decade working as an army doctor, where he became interested in epidemiology; this work would directly lead to his doctoral thesis on Hepatitis A.
In 1946, Zhdanov was invited to become Chief of the Epidemiology Department of the I. I. Mechnikoff Institute of Epidemiology and Microbiology in Kharkiv, becoming its director two years later. His work in virus classification saw him admitted to the International Committee on Taxonomy of Viruses as a life member.
Alice Bukrinskaya, the D.I. Ivanovsky Institute of Virology, Moscow, U.S.S.R, [3] would say this about him after he died:
Zhdanov was a scientist of keen and inquisitive mind and extraordinary flexibility of thought, a declared enemy of dogmatism and bureaucracy. He was an amiable, warm and charming person, highly appreciative of the other’s talent, a reliable, humorous and attentive friend.
In 1958, Zhdanov, as Deputy Minister of Health for the Soviet Union, called on the World Health Assembly to undertake a global initiative to eradicate smallpox. The proposal (Resolution WHA11.54) was accepted in 1959. Zhdanov left the Ministry of Health in 1961, and focused on scientific research for the rest of his career. This work included studying influenza, hepatitis, and in the 1980s, HIV.
In addition to his accomplishments in the field of public health, Zhdanov chaired the Soviet Union’s Interagency Science and Technology Council on Molecular Biology and Genetics, which among its many functions directed the Soviet biological weapons program.
Despite Zhdanov’s relative obscurity, some have argued that Zhdanov has done “more good for humanity” than any other human in history.[1]
To provide a rapid means of disseminating new findings in virology, Zhdanov founded the journal Problems in Virology in 1956; he remained its editor and an active contributor until his death.
Zhdanov’s years in health administration were marked by constant struggles with party officials on the Central Committee, who neither understood nor supported his initiatives and succeeded in blocking many of them. Unsatisfied and disillusioned with administrative work, Zhdanov left the Ministry of Health in 1961 to devote himself to scientific research. Because of his belief in the value of international cooperation, Zhdanov maintained close working associations with scientists in the West, even during the Corld War. Joint influenza research projects were done with Walter Dowdle, Robert Webster, Edward Kilbourne, and Nancy Cox; joint viral oncogenesis research projects were done with John Moloney and Fred Rapp; joint viral hepatitis research projects were done with Daniel Bradley and James Maynard, to mention but a few. [3]
References:
- Viktor Zhdanov, in Wikipedia. Accessed 13 February 2017
- World Health Assembly Resolution WHA11.54
- In memory of Victor Zhdanov. Archives of Virology 1991, Volume 121, Issue 1-4, pp 237-240
A day to remember: John Enders

John Enders
It was 120 years ago, today
John Franklin Enders (February 10, 1897 – September 8, 1985) was an American biomedical scientist and Nobel laureate. Enders has been called
“The Father of Modern Vaccines.”
In 1949, Enders, Thomas Huckle Weller, and Frederick Chapman Robbins reported successful in vitro culture of an animal virus—poliovirus. The three received the 1954 Nobel Prize in Physiology or Medicine “for their discovery of the ability of polioviruses to grow in cultures of various types of tissue”.
Meanwhile, Jonas Salk applied the Enders-Weller-Robbins technique to produce large quantities of poliovirus, and then developed a polio vaccine in 1952. Upon the 1954 polio vaccine field trial, whose success Salk announced on the radio, Salk became a public hero but failed to credit the many other researchers that his effort rode upon, and was somewhat shunned by America’s scientific establishment.
In 1954, Enders and Peebles isolated measlesvirus from an 11-year-old boy, David Edmonston. Enders began development of measles vaccine, using the ‘Edmonston-B Strain’ of the virus, leading up to the ‘Enders-Edmonston Strain’ for the vaccine. In October 1960, an Enders team began trials on 1,500 mentally retarded children in New York City and on 4,000 children in Nigeria.
On 17 September 1961, New York Times announced the measles vaccine effective. Refusing credit for only himself, Enders stressed the collaborative nature of the effort. In 1963, Pfizer introduced a deactivated measles vaccine, and Merck & Co introduced an attenuated measles vaccine.
References
A day to remember: Kiyoshi Shiga

Kiyoshi Shiga
Kiyoshi Shiga (February 7, 1871 – January 25, 1957) was a Japanese physician and bacteriologist.
Shiga was born in Sendai, Miyagi Prefecture, though his original family name was Satō. He graduated from the Medical School of Tokyo Imperial University in 1896 and went to work at the Institute for the Study of Infectious Diseases under Dr. Kitasato Shibasaburō. Shiga became famous for the discovery of Shigella dysenteriae, the bacillus causing dysentery, in 1897, during a severe epidemic in which more than 90,000 cases were reported, with a mortality rate approaching 30%.[1] The bacterium Shigella was thus named after him, as well as the shiga toxin, which is produced by the bacterium.
When attending the trecentenary celebration at Harvard [2], 1936, he opened his speech with:
Many thousands still suffer from this disease every year, and the light of hope that once burned so brightly has faded as a dream of a summer night. This sacred fire must not burn out
After the discovery of Shigella, Shiga worked with Paul Ehrlich in Germany from 1901 to 1905. After returning to Japan, he resumed the study of infectious diseases with Dr. Kitasato. He became a professor at Keio University in 1920.
From 1929 to 1931, Shiga was the president of Keijō Imperial University in Keijo (Seoul) and was senior medical advisor to the Japanese Governor-General of Korea. Shiga was a recipient of the Order of Culture in 1944. He was also awarded the Order of the Sacred Treasure, 1st class, on his death in 1957.
Shigellosis
The causative agent of human shigellosis, Shigella causes disease in primates, but not in other mammals. It is only naturally found in humans and gorillas. During infection, it typically causes dysentery.
Shigella is one of the leading bacterial causes of diarrhea worldwide, causing an estimated 80-165 million cases. The number of deaths it causes each year is estimated at between 74,000 and 600,000 deaths. It is in the top four pathogens that cause moderate-to-severe diarrhea in African and South Asian children.
References
Transmissible Briefs: Podcast channel for learning
Do you find it challenging to keep up with reading all public health news that is published daily?
With this new channel we aim to alleviate the task just a tiny bit. By regularly selecting European public health news in communicable disease control, Briefs is a channel to summarise reports on public health alerts and to combine it with a brief recap of key messages about our knowledge of the pathogen.
You can subscribe to Briefs as a Podcast, via iTunes or simply tap into the feed.
In addition, you can watch it on Vimeo:
[vimeography id=”1″]
Hope you like it 🙂
A day to remember – Jaume Ferran i Clua

Jaume Ferran
Jaume Ferran i Clua (Corbera d’Ebre, 1851 – Barcelona 1929) was a Spanish bacteriologist and sanitarian, contemporary of Koch, and said by his fellows to have made some of the discoveries attributed to Koch. As early as 1885, he wrote on immunization against cholera. In 1893, his work on this subject was translated into French with the title L’Inoculation préventive contre le Cholera.
Tuberculosis is another disease in which Ferran was always deeply interested.
As the son of the village doctor, he graduated in Medicine at the University of Barcelona. There he became interested in bacteriology. In 1884, the Royal Academy of Medicine recognized his report on ‘bacterial parasitism’ and the Barcelona municipality sent him to Marseilles to study the epidemic of cholera. Convinced of its bacterial etiology, newly discovered by Koch, Ferran prepared attenuated cultures of the vibrio bacillus, which was used to develop the first cholera vaccine.
During the outbreak of epidemic cholera in 1885, Ferrán was called to Valencia , to organise mass inoculation of the population. In spite of the success obtained, a controversy was unleashed; some believed that the Ferranian method was dangerous, and subsequently, the Government prohibited vaccination.
Later, he carried out research on the immunology of abdominal typhus and diphtheria and proposed a supra-intensive method for anti-rabies vaccination.

On the historic Lazareto in the middle of the port of Mahon at Menorca, Spain, you may find an expressive bronze statue of a man who breaks away from chains. Engraved on the four sides of the stone plinth are the names of four eminent men of medicine and medical science, of microbiology, of bacteriology and of immunology: Louis Pasteur, (France), Juan Carlos Finlay (Cuban), Sir Alexander Fleming, (Scottish) and Edward Jenner (English). It is an appreciation to pioneers who have furthered our knowledge about infectious diseases, in an era that still relied on quarantine islands such as the Lazareto of Mahon.
References:
- Jaume Ferran i Clua, in Wikipedia. https://en.wikipedia.org/wiki/Jaume_Ferran_i_Clua. accessed 31 january 2017.
- Discover Lazareto, Menorca’s Historic Treasure. http://menorca-live.com/discover-lazareto-menorcas-historic-treasure/
A day to remember – Baron Kitasato Shibasaburo

Kitasato Shibasaburō
Baron Kitasato Shibasaburō (January 29, 1853 – June 13, 1931) was a Japanese physician and bacteriologist during the prewar period. [1] He is remembered as the co-discoverer of the infectious agent of bubonic plague in Hong Kong in 1894, almost simultaneously with Alexandre Yersin. [3,4]
Kitasato was nominated for the Nobel Prize in Physiology or Medicine in 1901. Kitasato and Emil von Behring working together in Berlin in 1890 announced the discovery of diphtheria antitoxin serum, Von Behring was awarded the 1901 Nobel Prize because of this work, but Kitasato was not.
Kitasato was born in Okuni village, Higo Province, (present-day Oguni Town, Kumamoto Prefecture, Kyūshū). He studied under Dr. Robert Koch in the University of Berlin from 1885 to 1891. In 1889, he was the first person to grow the Tetanus bacillus in pure culture, and in 1890 cooperated with Emil von Behring in developing a serum therapy for tetanus using this pure culture. He also worked on antitoxins for diphtheria and anthrax. Kitasato and Behring demonstrated the value of antitoxin in preventing disease by producing a passive immunity to tetanus in an animal that received graded injections of blood serum from another animal infected with the disease.
After returning to Japan in 1891 he founded the Institute for Study of Infectious Diseases with the assistance of Fukuzawa Yukichi. One of his early assistants was August von Wassermann. Kitasato demonstrated how dead cultures can be used in vaccination. He also studied the mode of infection in tuberculosis.
He traveled to Hong Kong in 1894 at the request of the Japanese government during an outbreak of the bubonic plague, and identified a bacterium that he concluded was causing the disease. Yersin, working separately, found the same organism several days later. Because Kitasato’s initial reports were vague and somewhat contradictory, some scientific historians give Yersin sole credit for the discovery, while others advise dual credit, however, a thorough analysis of the morphology of the organism discovered by Kitasato by microbiologists determined that although his samples likely became contaminated later, leading to the conflicting reports from his laboratory, there is “little doubt that Kitasato did isolate, study, and reasonably characterize the plague bacillus” in Hong Kong and “should not be denied this credit”.[2]
Four years later, Kitasato and his student Shiga Kiyoshi were able to isolate and describe the organism that caused dysentery.
When the Institute for Infectious Diseases was incorporated into Tokyo Imperial University in 1914, he resigned in protest and founded the Kitasato Institute (the forerunner of Kitasato University), which he headed for the rest of his life.
In September 1921 Kitasato founded, together with several medical scientists, the Sekisen Ken-onki Corporation with the intention of manufacturing the most reliable clinical thermometer possible. The company was later renamed Terumo Corporation.
Kitasato also was the first dean of Medicine at Keio University, first president of the Japan Medical Association, and served on the House of Peers. He was ennobled with the title of danshaku (baron) in the kazoku peerage system in February 1924.
Kitasato died of an Intracranial hemorrhage at his home in Azabu, Tokyo on June 13, 1931. His grave is at the Aoyama Cemetery in Tokyo.
References:
- Kitasato Shibasaburō in Wikipedia. https://en.wikipedia.org/wiki/Kitasato Shibasaburō. Accessed 25 January 2017.
- Bibel, DJ; Chen, TH (September 1976). “Diagnosis of plaque: an analysis of the Yersin-Kitasato controversy.”. Bacteriological Reviews. 40 (3): 633–651, quote p. 646. PMC 413974. Freely accessible. PMID 10879.
- E.G. Pryor. The Great plague of Hongkong.
- Medical report on the epidemic of bubonic plague 1894.
Result of Poll ‘Most important public health publication’
On Januari 10 we posted a small poll on the Facebook page: ‘what do you consider the most important public health publication ever?’
Two weeks later we closed the poll and count the submissions. The poll reached 11.164 Facebook users, received 238 likes, and harvested 12 submissions of publication titles. Two titles received most votes, and seven additional titles were submitted only once. These are the results:
- The Mortality of Doctors in Relation to Their Smoking Habits (Richard Doll and A Bradford Hill, 1954) – most votes
- The etiology, concept, and prophylaxis of childbed fever (Ignaz Semmelweis,1861) – runner up
Other publications proposed once:
- Epidemics I, II and III by Hipppocrates
- The English Public Health Act 1848 (By E. Chadwick)
- An Inquiry Into the Causes and Effects of the Variolæ Vaccinæ, Or Cow-Pox. (Edward Jenner, 1798)
- An Essay on the Most Effectual Means of Preserving the Health of Seamen (James Lind, 1762)
- The Framingham Study: the epidemiology of atherosclerotic disease (Dawber, 1980)
- Publications on the North Karelia Project (Finland, started 1972).
- On the communication of cholera by impure Thames water (John Snow, 1854)
Reflections
No publications from the last 35 years were proposed, even though the people submitting to the poll are clearly of the ‘modern generation’. What does this mean? Did we achieve all major public health progress? Perhaps… Still, there is lots to do in my view.
In addition, it seems to me that many of the proposed publications have been at the centre point of much scientific and social controversy. The Doll and Hill study provided convincing evidence of association, yet the causality would be debated for decades. In fact, Bradford Hill formulated ‘viewpoints on causality’ (not ‘criteria’, mind you), where he described the challenges of attributing causality very well.
I wonder what Sir Bradford Hill would say of the current trend in society to ignore facts, and embrace ‘alternative facts’
The runner up, an impressive set of accurate observations of an intelligent young doctor, has never in his lifetime provided him with the credit due. Ignaz Semmelweis was branded a fool, and was committed to a mental hospital, where he died a miserable death. And most of us are well aware of the scepsis that John Snow had to confront, with his theory of waterborne spread of cholera in the mid-nineteenth century. At least his findings were strengthened thanks to his most fierce opponent, William Farr, who was determined to prove that Snow’s conclusions where incorrect. Farr ended up proving independently, that Snow’s conclusions made sense statistically.
The number of submissions is lower than I had expected, especially considering the wide distribution of this poll (over 10.000 readers). Yet I have to admit to have expected at least one reference to civil engineering, a professional field with profound impact on primordial prevention of communicable diseases in modern society.
Finally, it was a pleasant surprise to see the Public Health Act by Edward Chadwick (1848) as a proposed candidate. I agree that scientific, peer-reviewed publications, though of essential importance, are the sole achievement of public health progress, contrary to what some scientific institutes may wish to believe. And it is a nice timing, considering we could celebrate the 216th birthday of Chadwick yesterday.
Conclusion
The public health value of the submitted titles is undisputed, and it is good to see how much our current generation is still aware of this. Still, in the recent four decades, much public health impact has been achieved, and I wonder if there is not yet a contender to the title ‘most valuable public health publication’, that we have missed in this poll.
If we did, please let me know.
New podcast launched – Candida auris
T oday, a new podcast is released in the series Transmissible Briefs. It summarizes the recent ECDC Rapid Risk Assessment for Candida auris, published just before christmas 2016.
oday, a new podcast is released in the series Transmissible Briefs. It summarizes the recent ECDC Rapid Risk Assessment for Candida auris, published just before christmas 2016.
The series of podcasts aims to post at least once a month, on a topic of recent public health events. You can subscribe to the series using our RSS feed. If you prefer, it can also be followed on Vimeo. Feel free to comment and share feedback on this post and on the podcast.
Hope you will enjoy.
A day to remember: Edward Chadwick
Today it is 216 years ago that Edwin Chadwick was born in Manchester.
Sir Edwin Chadwick (24 January 1800 – 6 July 1890) was an English social reformer who is noted for his work to reform the Poor Laws and to improve sanitation and public health. He was born in 1800 at Longsight, Manchester, to James Chadwick. His mother died when he was still a young child, yet to be named. His father, James Chadwick, tutored the scientist John Dalton in music and botany and was considered an advanced liberal politician, thus exposing young Edwin to political and social ideas. [1]
At 18, he decided to pursue a career in law and undertook an apprenticeship at an attorney’s office. In 1823, he enrolled in law school at The Temple in London. On 26 November 1830 he was called to the bar, becoming a barrister, also known as a court lawyer.
Called to the bar without independent means, he sought to support himself by literary work such as his work on Applied Science and its Place in Democracy, and his essays in the Westminster Review, mainly on different methods of applying scientific knowledge to the practice of government. He became friends with two of the leading philosophers of the day, John Stuart Mill and Jeremy Bentham. Bentham engaged him as a literary assistant and left him a large legacy. He also became acquaintances with Thomas Southwood Smith, Neil Arnott, and James Kay-Shuttleworth, all doctors.
From his exposure to social reform and under the influence of his friends, he began to devote his efforts to sanitary reform. While still officially working with the Poor Law, Chadwick took up the question of sanitation in conjunction with Dr Thomas Southwood Smith. Their joint efforts produced a salutary improvement in the public health. His report on The Sanitary Condition of the Labouring Population (1842) was researched and published at his own expense. A supplementary report was also published in 1843. The formation of the Health of Towns Association and the creation of various city-based branches followed rapidly. The national and local movements contributed to the passing of the Public Health Act 1848. [1]
By 1848 Chadwick had become Sanitary Commissioner of London, and was very influential in the city’s approach towards cholera. He believed that filth in rivers was less dangerous than filth in sewers. As Commissioner, he had the power to have sewers regularly flushed into the River Thames. This policy inadvertently contributed to the spread of cholera by water purveyors which had their intakes in the polluted areas of the river. Contrary to Dr. John Snow, he was a strong believer in the theory that epidemics were generated spontaneously from dirt, and that basic sanitation rather than specific avoidance of cholera germs would control the disease. He rejected with scorn as mere hypothesis Snow’s germ theory, as described in Snow’s 1855 book. [2]
While others had come to accept the germ theory, Chadwick remained a committed sanitarian to the end, telling a newspaper reporter shortly before his death in 1890:
“I cannot tell you how strongly I believe in soap and water as a preventive of epidemics” (Weekly Dispatch, July 13, 1890).
It is interesting to observe that this strong position on use of soap and water, is not at all in conflict with the germ theory. In addition, his work on mapping disease in Bethnal Green Parish resembles the later work of Snow on mapping cholera mortality a lot. It seems that the two men had a strong passion for improving public health in common, and were mainly divided over the theories of causation.
In January 1884, he was appointed as the first president of the Association of Public Sanitary Inspectors, now the Chartered Institute of Environmental Health. Its head office, in Waterloo, London, is named Chadwick Court, in his honour.
In recognition of his public service, he was knighted in 1889. He served in his post until his death, at 90, in 1890, at East Sheen, Surrey.
References:
A day to remember – Gertrude Mary Cox

Gertrude May Cox
Anyone who uses statistics, or who is involved in study design, should know who Gertrude Cox was. To be clear: she did not give her name to Cox regression (proportional hazards model). That was Sir David Cox, from Birmingham; ‘the other famous Cox-statistician’.
Born January 13, 1900, in Dayton, Iowa, Gertrude M. Cox reflected the upbringing of the times and location. It is said she was “instilled with ethics, moral courage, and determination”. Her professional efforts succeeded to make statistics practical for those working in agricultural and biological research. This achievement, together with her strong organizing skills, made her successfully bridge the gap between theoreticians and research workers.
After a bachelor studies in mathematics, she received a master’s degree in statistics from Iowa State College in 1931. She then studied psychological statistics and was a graduate assistant at the University of California, Berkeley. In 1933 she returned to Iowa to assist George Snedecor by heading the newly created Statistical Laboratory. Cox answered Snedecor’s call to work as a consultant and manage his Iowa State Statistics Lab of “computers” for the statistical consulting projects.
“Computers”, as they referred to them, were the women hired to compute sums of squares and standard deviations on big, cumbersome Merchant and Monroe desk calculators. Because of their attention to detail, women were often sought for this task.
Cox was made a research assistant professor in 1939. During this period she began her research on experimental design. During this period she compiled a series of notes on standard designs, that eventually led to the book ‘Experimental Designs’, cowritten by William G. Cochran and published in 1950.
Her appointment in 1940 to organize and head a Department of Experimental Statistics in the School of Agriculture at North Carolina State College in Raleigh, seemed to be the result of a footnote in a letter from Snedecor to North Carolina State College, in which he recommended five men. He added,
“Of course if you would consider a woman for this position I would recommend Gertrude Cox of my staff.”
In January 1941, the department was established with Cox as the first female full professor and first female department head at North Carolina State College, a propitious choice that changed the course of statistics in North Carolina.
Her skill as an administrator was unsurpassed. She employed outstanding faculty and staff and left them to their teaching and research while she raised funds. In addition to her administrative duties, Cox continued to teach, drawing on her many years of consulting to produce practical real-life examples designed to illustrate experimental designs, all of which were flawlessly computed before the age of computers.
She held many positions that were of great influence on the field of statistics. Among many examples, she was the first woman to be elected president (1956) of the American Statistical Association (founded in 1839).
In addition to her professional achievements, Cox was known for the personal interest she took in relatives, friends, and wives and children of faculty and staff. The memory books in the Department of Statistics at North Carolina State University hold many remembrances of her tenure there, from newspaper clippings of awards to the department to wedding invitations for staff and Christmas cards sent and received.
Cox died of leukemia October 17, 1978.
References
Today’s Patron Saint – St. Adelard of Corbie

St. Adelard of Corbie, patron saint of typhus sufferers.
Adelard (752-827) was the grandson of Charles Martel, nephew of King Pepin and first cousin to Charlemagne. He became a monk, at the age of 20, at Corbie in Picardy in 773. Eventually he was chosen abbot, and became Charlemagne’s counselor. He was forced by the king to quit the monastery and work for him as chief minister for his son Pepin. He was accused of supporting a rival power (Bernard) against Emperor Louis the Debonair and was banished to a monastery on the island of Heri. Five years later he was recalled to the king’s court (821). He later retired to the Abbey at Corbie and died January 2 after an illness. Miracles were reported after his death. When Adelard first became monk at Corby in Picardy (in 773), his first assignment was gardener of the monastery. He did his job humbly and piously, praying throughout the day. His great virtues eventually helped him become Abbot. [1]
Saint Adelard was canonized by Pope John XIX in 1026.
With the above description in mind, it will not be much of a surprise that Adelard became the patron saint of gardeners. In addition, he became known as the patron of sufferers of fevers and typhus. [2]
In this case it will be important to know that for a long time, the diagnose ‘typhus’ was used for all types of severe febrile illness. The Greek term [typhos] (smoke, mist, fog) was employed by Hippocrates to define a confused state of the intellect, with a tendency to stupor (stupor attonitus); and in this sense it is aptly applied to typhus fever with its slow cerebration and drowsy stupor. Boissier de Sauvages first (in 1760) called this fever “typhus,” and the name was adopted by Cullen of Edinburgh in 1769. Previous to the time of de Sauvages typhus was known as “Pestilential” or “Putrid Fever,” or by some name suggested by the eruption, or expressive of the locality in which it appeared, as “Camp,” “Jail,” “Hospital,” or “Ship Fever”. [3]
In modern medicine, typhus refers to a group of infectious diseases that are caused by rickettsial organisms and that result in an acute febrile illness. Arthropod vectors transmit the etiologic agents to humans. The principle diseases of this group are epidemic or louse-borne typhus (Typhus exanthematicus, classic typhus fever; caused by Rickettsia prowazekii) and its recrudescent form known as Brill-Zinsser disease, murine typhus (flea-borne typhus fever, Shop typhus; caused by Rickettsia typhi -Rickettsia mooseri-; R. felis), and scrub typhus (mite-borne typhus fever, Tsutsugamushi disease; caused by Orientia tsutsugamushi). [4]
References
- Adelard of Corbie. Catholic Online Website. http://www.catholic.org/saints/saint.php?saint_id=252. Accessed 2 January 2017.
- January 2, St. Adelard of Corbie – Patron saint of gardeners and sufferers of typhus. 365rosaries website. https://365rosaries.blogspot.nl/2013/01/january-2-2013-saint-adelard-of-corbie.html. Accessed 2 January 2017.
- Typhus. Online etymology dictionary. http://www.etymonline.com/index.php?term=typhus. Accessed 2 January 2017.
- David L. Heymann (ed). Control of Communicable Diseases Manual, 18th edition. Pages 630-6
Public Health Competencies – A perspective
50 years of ASPHER
The Association for Schools of Public Health in the European Region (ASPHER) celebrated the 50th birthday this year. Reason to celebrate. Also a good opportunity to look ahead, in an attempt to predict what new domains lay ahead in the future.
Invited by ASPHER, I wrote a perspective on public health competencies, the role of ASPHER until now and an expectation of future challenges. The result is published today in EuroHealth, the Quarterly Journal of the European Observatory of Health Systems and Policy.
For those who are interested, you may read the issue below:
[pdf-embedder url=”https://www.transmissible.eu/wp-content/uploads/2016/12/Eurohealth-V22-N4-2016.pdf”]
A day to remember: Emile Roux
 Pierre Paul Émile Roux (17 December 1853, Confolens, Charente – 3 November 1933, Paris) was a French physician, bacteriologist and immunologist. Roux was one of the closest collaborators of Louis Pasteur (1822–1895), a co-founder of the Pasteur Institute, and responsible for the Institute’s production of the anti-diphtheria serum, the first effective therapy for this disease.[1]
Pierre Paul Émile Roux (17 December 1853, Confolens, Charente – 3 November 1933, Paris) was a French physician, bacteriologist and immunologist. Roux was one of the closest collaborators of Louis Pasteur (1822–1895), a co-founder of the Pasteur Institute, and responsible for the Institute’s production of the anti-diphtheria serum, the first effective therapy for this disease.[1]
When the Institut Pasteur was created in 1888, Roux was placed in charge of instruction in microbiology. At the same time, he became director of the Service de Microbie Technique and began his most important original work. There, confirmed the pathological role of the diphtheria bacillus, and perhaps most important, he demonstrated with A. E. J. Yersin that the pathogenic power of this bacillus depends not merely on its presence but, rather, on a poison, or toxin, that it produces.
“snake venoms themselves are not as deadly.”
Roux said about this toxin.[2] Would his parents have been aware that the initials of their son (PPE) would become a key concept in communicable disease control?
References:
- Emile Roux, from Wikipedia, the free encyclopedia, accessed 17 december 2016
- Medicine Biographies-Emile Roux, Encyclopedia.com, accessed 17 december 2017
Want to see more “days to remember”? Take a look at our 2017 Year Planner.
[twitter_share text=”A day to remember: Birthday of Emile Roux, 17 december 1853″ url=”https://www.transmissible.eu/archives/1895″/]
A day to remember: Margaret Mead
 Margaret Mead (December 16, 1901 – November 15, 1978) was an American cultural anthropologist who featured frequently as an author and speaker in the mass media during the 1960s and 1970s. She earned her bachelor’s degree at Barnard College in New York City and her M.A. and Ph.D. degrees from Columbia University.
Margaret Mead (December 16, 1901 – November 15, 1978) was an American cultural anthropologist who featured frequently as an author and speaker in the mass media during the 1960s and 1970s. She earned her bachelor’s degree at Barnard College in New York City and her M.A. and Ph.D. degrees from Columbia University.
Mead was a respected and often controversial academic who popularized the insights of anthropology in modern American and Western culture. Her reports detailing the attitudes towards sex in South Pacific and Southeast Asian traditional cultures influenced the 1960s sexual revolution. She was a proponent of broadening sexual mores within a context of traditional Western religious life. [1]
When Margaret Mead died in 1978, she was the most famous anthropologist in the world. Indeed, it was through her work that many people learned about anthropology and its holistic vision of the human species. [2] And as such, she contributed a wealth of knowledge and insights to public health.
One of her quotes:
As the traveler who has once been from home is wiser than he who has never left his own doorstep, so a knowledge of one other culture should sharpen our ability to scrutinize more steadily, to appreciate more lovingly, our own.
December 16, the birthday of Margaret Mead, is a day to remember her.
References:
- Margaret Mead, from Wikipedia, the Free Encyclopedia, accessed on 15 December 2016
- Institute for Intercultural Studies: Margaret Mead, an anthropology of human freedom. Biography.
Want to see more “days to remember”? Take a look at our 2017 Year Planner.
Ready to plan 2017?

Transmissible’s 2017 Year Planner
Such lovely birthday present I got
The Transmissible Year Planner 2017 has arrived! In total, 102 historic Infectious Disease Icons and Saints appear on the planner. A quiz is incorporated in the margins of the planner; 17 descriptions to be linked to dates.
Curious for the details? Download the PDF here, for you to print out. Be aware that the planner requires to be printed on at least A2 size, preferably A1, in order to be useful as planning tool. However, printing it on A3 will probably allow you to read the fine print, and therefore have a go at solving the quiz. Link the descriptions to the correct dates and send the answers to Info@Transmissible.eu, including your name and address, and have a chance to win a prize:
The first 5 people who submit the correct answers will receive a full color A0 size year planner.
Full color printed version also available in our shop.
A sound mind in a sound body?
Mens sana in corpore sano – the Roman poet Decimus Iūnius Iuvenālis used these words in a poem, almost twenty centuries ago. Sounds good, no? A sound mind in a sound body.
Keeping dangerous microbes at a distance is a good strategy to stay healthy. Can sounds help us with that? Not sure. What we do know, is that there is a large volume of songs dedicated to some aspects of communicable diseases. And since December is a playful month, we thought it was a nice idea to set up a playlist with ‘Transmissible Sounds. You can subscribe to it on Spotify. Do you miss your favorite infectious disease song? Just add it below in the comments (only accessible for registered users)
Enjoy !
World AIDS Day
Time flies. ESCAIDE2016 has barely closed and #WorldAIDSDay already welcomes us.
I am curious for your ideas on health education for youth on HIV and AIDS in our current times. When I was trained in Public Health, our HIV Peer Educators spend much time browsing through the newspapers, scanning the contact adds and announcements of events where those at risk for HIV would participate. Few health messages were delivered through schools and social media as we know them, did not exist.
In a Twitter Poll, I ask the following:
To all followers of #WorldAidsDay, what do you consider the best channel to educate youth on HIV/AIDS?
— Transmissible (@iTrainEU) December 1, 2016
Please let me know your views and vote.
Thanks, and have a good WorldAIDSDay. Make a difference!
ESCAIDE 2016 is ongoing now
From 28-30 November 2016, the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE) is held again in Stockholm. This 10th edition has even more Twitter exposure as previously, so it is possible to follow the conference at a distance, using #ESCAIDE2016. This is great, since not all interested professionals are able to travel to Sweden to participate. Though presentations are not yet streamed live, as some other EU conferences do (look at how EFSA deals with this at their 2015 Scientific Conference), this is already a very good way to stay involved.
For those of you who want something to do between the Twitter feeds, here is another jigsaw from one of the ESCAIDE Tweets: Have fun !
(If you cannot see the entire puzzle, try clicking full screen, on the lower right corner)
Vaccine Narratives on Mommy Blogs: what machine-learning tells us
A fascinating paper appeared today in the Journal of Medical Internet Research:
“Mommy Blogs” and the Vaccination Exemption Narrative: Results From A Machine-Learning Approach for Story Aggregation on Parenting Social Media Sites.
by Timothy Tangherlini and colleagues.
The title is a mouth-full, but what did they do?
The researchers knew that social media offer great opportunities to explore how people talk about health care on a very large scale. They selected two websites where parents seek and discuss information about health: the so-called ‘Mommy Blogs’. They wanted to develop an automated machine-learning method to discover how parents talk about vaccines.
Looking at a data-set of 1.99 million posts, contributed by 40,056 users (and were viewed 20.12 million times) on two parenting websites, they determined first the topics of discussions. Then they extracted the underlying stories and story fragments, using techniques that impress me as mysterious: generative statistical-mechanical narrative models.
What did they find?
They found that a lot of stories were about exemption from vaccination requirements. Most narratives related to seeking exemption and to a culture of distrust of government and medical institutions. Discussions and stories showed that parents used religion or belief to get exemption from vaccination. Fear of adverse reactions played a key role, whether or not these reactions were truly associated with vaccines. Such stories persisted on the websites, even when parents left the discussion forms. In most vaccination stories from the sites they analysed, it was taken for granted that vaccines, and not vaccine preventable diseases, pose a threat to children. Because vaccines are seen as a threat, parents focus on sharing successful strategies for avoiding them. When new parents join such sites, they may be exposed to the ‘endemic narrative frameworks’ in the forums, which may influence their health decision making.
Considerations
This type of analysis is new to me. I knew that data mining and text mining techniques are being used increasingly in health care, this is the first article I read about an application to knowledge, attitudes and believes of parents about vaccination.
Social media play such an important role in daily life, and increasingly so, that this field of research seems to me a relevant addition to the field of public health science.
Read the full article here:
[pdf-embedder url=”http://publichealth.jmir.org/article/viewFile/publichealth_v2i2e166/2″]
Did you like this post? Let me know by liking our Facebook Page below. You can also share your comments and ideas about this post
The Vienna Declaration
First a brief review of public health history:
In November 1986, the World Health Organization organised the First International Conference on Health Promotion, in Ottawa. At this meeting, a Charter for Health Promotion was signed. This international agreement launched a series of actions among international organizations, national governments and local communities to achieve the goal of “Health For All” by the year 2000 and beyond through better health promotion. This Health for All target was already developed in the 70’ies, and recognises that every person on this planet needs and is entitled to the highest possible standard of health.
Three decades following the Ottawa Charter:
New and emerging threats to public health have arisen. So now, in November 2016, the European Public Health Association and the Austrian Public Health Association have set up the Vienna Declaration to emphasize their commitment to the principles of the Ottawa Charter on Health Promotion.
The Vienna Declaration extends and updates the Charter’s pre-requisites for health. When I look at them, it appears to me how obvious these requisites sound to someone like me, living in Northern Europe:
- Peace and freedom from fear of violence, including that within communities and families;
- Shelter that provides protection from the elements, a safe indoor environment, and access to basic utilities;
- Education for all, regardless of gender, sexuality and sexual identity, race, ethnicity, religion, and citizenship;
- Affordable, accessible, nutritious and healthy food;
- A living income, coupled with protection from fear of catastrophic expenditure and unaffordable debt;
- A stable, sustainable, and healthy eco-system, as free as possible from pollutants;
- Access to sustainable resources, especially energy sources and clean water;
- Social justice, equity, and empowerment for all, regardless of gender, sexuality and sexual identity, race, ethnicity, religion, and citizenship;
- Systems of local, national, regional, and global governance that are open and transparent, democratically accountable, and represent the interests of all members of their populations;
- Systems that provide high levels of social protection for all;
- Good quality work, with fair employment policies and safe, health-promoting working conditions;
- Optimal early childhood conditions, offering loving, supportive, responsive, nurturing and stimulating environments.
Looking at this from the perspective of global health, it is painfully clear that many of these important pre-requisites for health are absent in a lot of parts of the world.
Organisations and individuals can support the Vienna Declaration, by pledging to:
- develop, use, and improve inclusive, high-quality, transparent and innovative information systems that can inform public health policies;
- advocate for health, working with those whose goals we share, even though they may not yet be engaged in the quest for better health, but challenging those whose words and activities threaten health;
- make visible the health effects of policies in all sectors, holding those in power accountable for their actions in the quest for better health;
- create a motivated, highly qualified workforce who, in their many different roles and sectors, can contribute to improved health for the entire population.
From the perspective of Transmissible, I pledge to fully engage in activities that support and further the Vienna Declaration.
Want to download the declaration? Follow this link.
Winter is Coming, and this is how you prepare
If you are living, like me, on the northern hemisphere of our planet, then you know that winter is coming. Tomorrow, summer time daylight saving will end in many countries and darkness will take most of our day, until spring.
This will also be the season where Influenza viruses will circulate in abundance, some old, some new. If we are lucky, we have already met the old ones in previous years, and our immune system will recognize them. It’s the new ones we should be concerned with most. They emerge from the bodies of their hosts with a new appearance; a make over. When they infect their host, they will, in fact, mix and mingle with other flu viruses they meet. Because, make no mistake, people will get infected with more than one flu virus at a time. And we become a biological dating site, where new generations of virus are born and emerge to the world. Some of these new generations will just have a new coat, a new disguise to evade the immune memory of the population. But others, they will have mutated in a whole new type, recombined entire parts of their virus-parents, and some of these combinations no man alive may have encountered before.
And we become a biological dating site, where new generations of virus are born and emerge to the world.
You know what makes matters even worse? Humans are not the only host for flu virus. They can infect and multiply in birds and pigs too. So in most countries were birds, pigs and humans live close together, we offer massive biological dating sites for the influenza virus to generate new types, that the world has not seen before.
You think this only happens in China, in remote rural areas? Wrong. Take a look at the map below, made in 2003 in the Netherlands, where poultry (chickens) and pigs are packed with hundreds of thousands together on an industrial scale in productions farms. And the Netherlands is the most densely populated country of Europe. Seventeen million people living in close proximity with 100 million chickens and sixteen million pigs. An influenza virus’ paradise.
Distribution of poultry farms in the Netherlands (grey circles) and those infected with avian influenza (orange circles) in 2003. From: Vogelpest Epidemie 2003: gevolgen voor de volksgezondheid, an RIVM Report by A. Bosman et al, 2003
And do you think you can only be infected by another host with influenza by touching them at close distance? Wrong again. Look at this video in an article of the New England Journal of Medicine. It shows you how far a sneeze travels.

In addition, infected slime droplets from flu patients can stick to any surface that you and I can touch with our bare hands: door knobs, taxi cab seats, ticket counters at the train station, railings at the base ball or soccer stadium, the report card that your grand child brings home from school. You name it. The list goes on.
And the Netherlands is the most densely populated country of Europe. Seventeen million people living in close proximity with 100 million chickens and sixteen million pigs. An influenza virus’ paradise.
So, do we hide? Do we stock our basement with food for 22 weeks – because that is how long the flu season can last each year – and barricade our doors?
No. We vaccinate.
Flu vaccination is our best way of protecting against the new strains that emerge each year. While we enjoy summer on the northern hemisphere, influenza specialists all around the globe hunt for new types and flu virus with new wardrobes on the southern hemisphere. During the southern winter, they trace, catch and harvest these new flu viruses and use them to produce a new generation of vaccines, able to protect vulnerable people from these new threats.
Every year.
And those vaccines offer the best chance to protect our vulnerable people – the very old, the very young, those with cardiac diseases, diabetes, kidney diseases, lung diseases etcetera – from an infection that could devastate their lives, or kill.
And now you may think. “Ah, so this is only risky for the vulnerable. I am healthy, so I do not need this vaccine”. Wrong again. Though it is true that healthy adults have a much lower risk of dying from a new influenza infection, it still happens. Or hospitalisation, even intensive care.
But the most pressing reason for healthy adults to vaccinate is the vulnerable group around them. Parents and grand parents, or patients. This is why Health Care Workers have duty to take flu shots every year. This is not only a medical duty, it is a moral one too, as Dr. Stanley Plotkin tells us in the excellent e-course on seasonal flu vaccination, freely available at the ECDC Virtual Academy.
So Winter is coming, and I know what I am going to do to prepare myself.
Do you?
Assessment of Needs, Requirements & Capacity for Training in Surveillance
In September and October, a team of Malawi public health experts performed an assessment of needs, requirements and capacity for training in surveillance in the country. I was hired by WHO as consultant to develop an assessment tool to support this process and the assessment team in Malawi. During a workshop of 3 days in October, I joined the team in Malawi to draft a plan of action for training the surveillance workforce. We reviewed the results and recommendations of the assessment, and selected training activities to build a stronger capacity for surveillance in Malawi.
The story of Polio in America
The latest Transmissible purchase: a documentary on poliomyelitis in America. Stream it here:
[arve url=”https://player.vimeo.com/video/107179865″ align=”center”]
The polio epidemic in early 20th century America was a crippling (literally) health threat that seized the world. This film, Babies and Breadwinners, documents the polio vaccination campaign in Columbus, Georgia. The film is a wonderful look at a typical 1960s community and how it was able to band together in order to fight off a menacing disease. Precious footage of all the different aspects of community are represented: the church, the city council and government, the schools, and even print and television ads. Most interestingly, an early shot of Bozo the Clown being vaccinated is included; nowadays this would not be used to promote vaccination given the hype of the horror clowns.
Babies and Breadwinners is a remarkable film that well documents the fight against polio as well as 60s community life.
Dead Birds – Mind the Knowledge Gap
Mid september 2016, the Dutch Wildlife Health Centre reported that Usutu virus causes mortality among blackbirds in the Netherlands. Reports about dead birds started to increase since August, mainly blackbirds and owls. The usutu virus (USUV) is an Arbovirus that has been observed in Europe during the past decennia. Mosquitoes vector the virus between different birds and only rarely infect humans; only five humans have been described with an usutu virus infection, three of them were immunocompromised. In 2010 ECDC reports human case with usutu virus infection. In 2014, ECDC releases technical guidelines for surveillance of native mosquitoes in Europe, including information about the three mosquito species that can act as vectors of usutu virus in Europe.
Mapping the Gap
Though the virus is known for decades, there are still many unknowns. In 2011 Eurosurveillance published an article on USUV and the potential risk for human disease. Still the natural history of human infection is largely unknown. In a serological study among blood donors, around 1% had antibodies against USUV. What is the risk of infection after an infected mosquito bite? Can asymptomatic people transmit the virus?.
These are not merely questions of academic interest. Effective prevention and control measures depend on detailed understanding of the chain of transmission from the natural reservoir of a pathogen, specific sources, and transmission routes in the external environment to strengthening immune defenses and reducing complications of the infection.
Intervention Epidemiology and Public Health Microbiology are disciplines with professionals trained to find answers to these questions and close the gap. Interested in Continuing Professional Education in those areas? Request information about our short courses; they can be organised for entire teams in your organisation.
Inspiring public health animated video examples
This week I came across the Defeat Diarrheal Diseases YouTube channel and immediately loved their approach. The use of audiovisuals and humor to get a basic yet oh so important public health message across. Here are two examples of their work. The third video is from GOOD Magazine, conveying the health risks of dirty water. Three nice and inspiring examples.
[iframe id=”https://www.youtube.com/embed/8iIvsgD2CP0″ align=”center” mode=”normal” autoplay=”yes”] [iframe id=”https://www.youtube.com/embed/80POTZN88Ho” align=”center” mode=”normal” autoplay=”no”] [iframe id=”https://www.youtube.com/embed/VE1bjbGj8XA” align=”center” mode=”normal” autoplay=”no”]
Table Top Simulation Exercise on Meningitis Outbreak
 On 1 September we contributed to an engaging Simulation Exercise with 5 syndicates in the NSPOH Summer School for Public Health. Players worked hard to get on top of controlling the rapidly evolving outbreak. Local, National and International aspects had to be dealt with. Compliments to all players: challenging setting, difficult to get the full picture. You have made an impressive press conference. Special mentioning for the creative press syndicate.
On 1 September we contributed to an engaging Simulation Exercise with 5 syndicates in the NSPOH Summer School for Public Health. Players worked hard to get on top of controlling the rapidly evolving outbreak. Local, National and International aspects had to be dealt with. Compliments to all players: challenging setting, difficult to get the full picture. You have made an impressive press conference. Special mentioning for the creative press syndicate.
Thanks to the NSPOH team for wonderful team work 🙂
What makes a teacher remarkable?
During the Kick-Off event on Juli 1, 2016, the visitors used Socrative Student to answer three questions. The posts of July 2 on ‘Documentaries that matter‘ and ‘What games make you keep playing?‘ gave an overview of the answers to the first two questions.
The third question asked the Kick-Off event vistors to think back of a teacher that they considered “very good”. They explained why they thought this was a good teacher. The answers of the 21 vistors had many elements in common.
Credibility and knowledge are obvious, basic elements, yet many answers indicated that a great teacher ‘bridges personal experience to academic theory’. This probably links to the element of credibility; the ability to demonstrate the practical value of theory. It requires story-telling skills, something that many respondents mentioned: the great teachers they remembered were all excellent and convincing story-tellers in some way. They ‘make the subject come alive’.
Another key element of a great teacher, that almost all respondents mentioned was ‘engaging’. The first step is to take students serious, independent of their age and experience. The next one is to engage by provoking their curiosity or trigger responses. This could be a geology teacher, taking students on a field trip in the mountains, pointing at the soil and asking “what the hell happened here 100.000 years ago?”. Or someone that moderates a good debate among the students, highlighting different points of view, without taking sides, yet still bringing it all together using facts, knowledge and experience.
Finally, in addition to the knowledge and skills, many people mention something about the attitude that makes a great teacher. It has to be positive, supportive, open and dedicated. Someone who ‘rejoices in the success of the students’ has a key factor in being an excellent teacher, according to one answer. Being open as well as challenging, is another description.
“They connect with the students, they inspire you […] and make the subject come alive”, “you can argue a point with them, without feeling they are looking down on you for daring to challenge what they think.”
It may be self-evident, yet all of the above is also about adaptability. Thank you very much, all participants to the Transmissible Kick-off, for providing such a valuable feedback and advice.

New crossword
Yes, we have a new one. Not difficult: the words to find are beside the puzzle. How fast can you find them? Submit your result when you think you are the fastest (requires registration and login).
HAVE FUN!
(oh, just press “play” ![]() to show the puzzle and start the clock)
to show the puzzle and start the clock)
[crosswordsearch mode=”solve” project=”Bugs” name=”Field Epi” timer=”0″ submitting=”1″]


